Technology Type
- Type
- Alkylation into High-Octane Alkylates
- Process
- Fuel Processes
- Abbreviation
- SAAU, HFAU
-
Alkylation Process in the Refinery
Alkylation is an important Refining Process for the Production of Alkylate, a High-Octane Gasoline Blending Component. Alkylate Product is a Mixture of Branched Paraffinic Hydrocarbons of Gasoline boiling range (mostly Isoheptane and Isooctane). Alkylate has a Motor Octane (MON) of 90-95 and a Research Octane (RON) of 93-98. Because of its high Octane and low vapor pressure, Alkylate is considered an excellent Blending Component for Gasoline.[1],[2]
In the Refinery, Isobutane is alkylated with Isobutene in the presence of a strong Acid Catalyst, either Sulfuric Acid or Hydrofluoric Acid. The Process is referred to as a SAAU or a HFAU. However, Oil Refinery Employees may simply refer to the Unit as the Alkyl or Alky Unit. Propylene present in the feed in small concentration also reacts with Isobutane to form Isoheptane.[1],[2]
Figure 1 - Principal Alkylation Reactions[3]
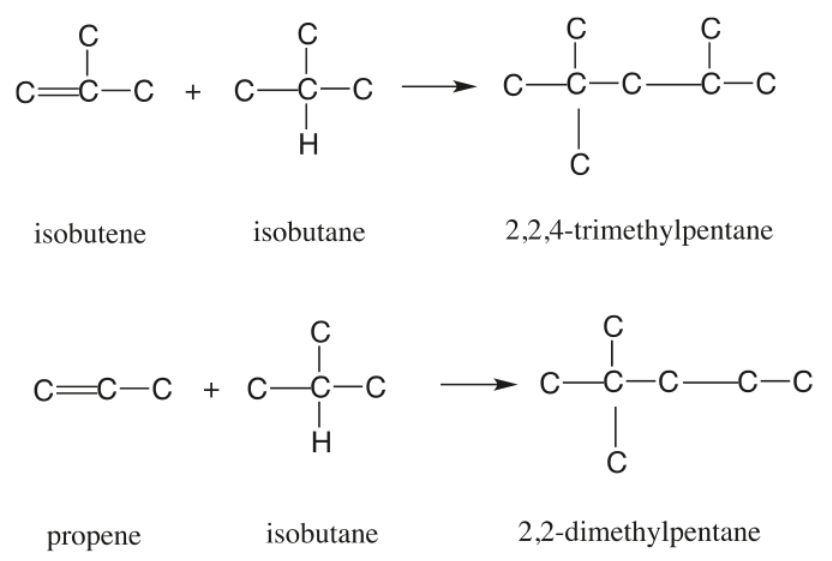
Under Reaction conditions unfavorable to Alkylate formation, Propylene may also Polymerize to form the undesirable Product Polypropylene. Amylenes can undergo a similar Reaction to form Alkylate, but since Amylenes have a high octane number to start with, their conversion to Alkylate is not as advantageous, as in the case of Butylenes. Another Side Reaction that can negatively affect the formation of Alkylate is Ester formation by the Reaction of Olefin with Sulfuric Acid.[1]
H2SO4 Alkylation Process Description
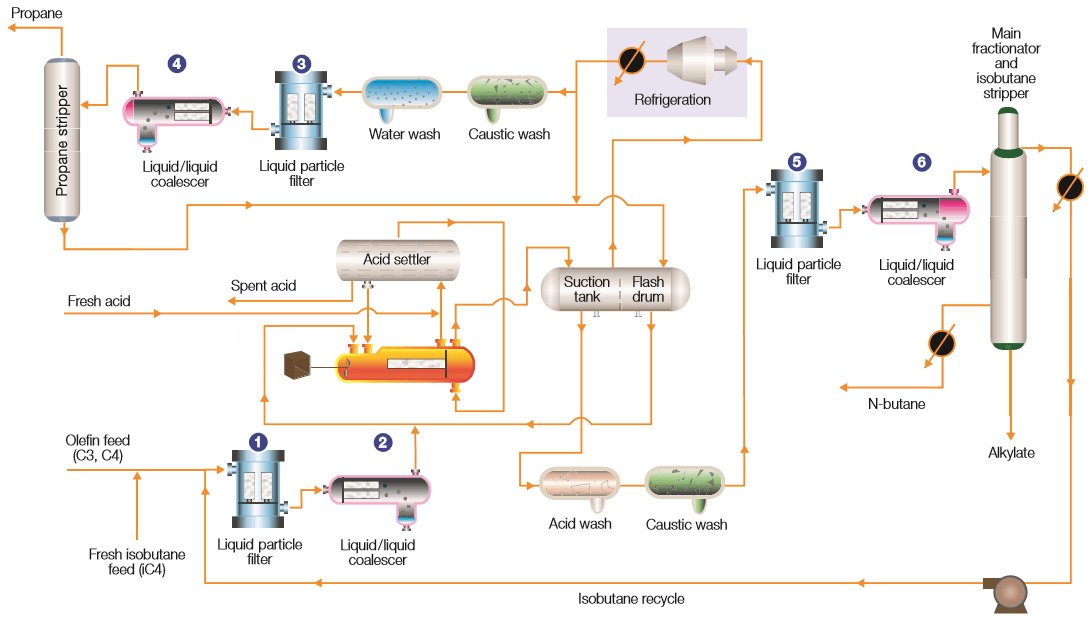
Olefins (C3-C4) from the FCC Unit are combined with Isobutane (i-C4). Concentrated Sulfuric Acid (H2SO4) is then mixed in and fed to the Reactor that is operated with Refrigeration to minimize the Formation of By-Products. The Alkylation reaction takes place, combining the Olefins with the Isobutane to form the Alkylate Product. The Hydrocarbons leaving the Reactor mostly consist of Alkylate, unreacted Isobutane, n-Butane and Propane.
The Acid and Alkylate Products are separated by a Settler and the Acid is recovered for Re-Use in the Reactor. The Reactor Hydrocarbons are separated by the Main Fractionator where the Isobutane is recycled to the Reactor, Alkylate is drawn from the Bottom, and an n-Butane Product Stream is separated from a Side Stream.
The Light Ends of the Process Fluids are used in the Refrigeration Loop and a Side Stream is passed through a Propane Stripper to recover Propane. Effective Separation is a critical component of an efficient, reliable, and safe Sulfuric Acid Alkylation Process.[4]
HF Alkylation Process
In the Hydrofluoric Acid Alkylation Process the HF Catalyst protonates the Alkenes (Propylene, Butylene) to produce reactive Carbocations, which alkylate Isobutane. The Reaction is carried out at mild temperatures (0 and 30 °C) in a Two-Phase Reaction.[5]
Commercial Technologies
Hydrofluoric Acid Catalyst Alkylation Technologies
- Honeywell UOP - HF Alkylation Process
- Lummus Technologies$ - HF Alkylation Process
$formerly ABB and Conoco Phillips
Sulfuric Acid Catalyst Alkylation Technologies
- Elessent Clean Technologies* - STRATCO®
- Elessent Clean Technologies* - ALKYSAFE® (Revamp of HF Alky Plants)
- ExxonMobil - ALKEMAX™
- Lummus Technologies - CDAlky®
*formerly DuPont Clean Technologies
Ionic Liquid Catalyst Alkylation Technologies
- Honeywell UOP - ISOALKY™
Solid Acid Catalyst Alkylation Technologies
- Air Liquide - LURGI EUROFUEL®
- KBR - K-SAAT™
- Honeywell UOP - Alkylene™
- Lummus Technologies# - AlkyClean®
- Topsoe FBA™
#formerly Akzo Nobel & ABB
1. Alkylation, Brewiki
2. REFINING - ALKYLATION, techSTAR
3. Dr. Semih Eser © Penn State, FSC 432 PETROLEUM PROCESSING, Alkylation, image licensed under CC BY-NC-SA 4.0
4. H2SO4 Alkylation Process Description, PALL
5. Hydrofluoric Acid, Wikipedia
- Link
System Info
- Updated by
-
 Kokel, Nicolas
Kokel, Nicolas - Updated
- 2/26/2023 5:35 PM
- Added
- 11/28/2022 2:05 PM

No Services yet available.
Enquire in Solutions how we can help you.
Technology Type Communicator
| Title | Date |
|---|
Image
Technologies
| Technology | Owner | |
|---|---|---|

|
Elessent | |

|
Exxon Chemical UK | |

|
Lummus | |

|
Honeywell UOP |