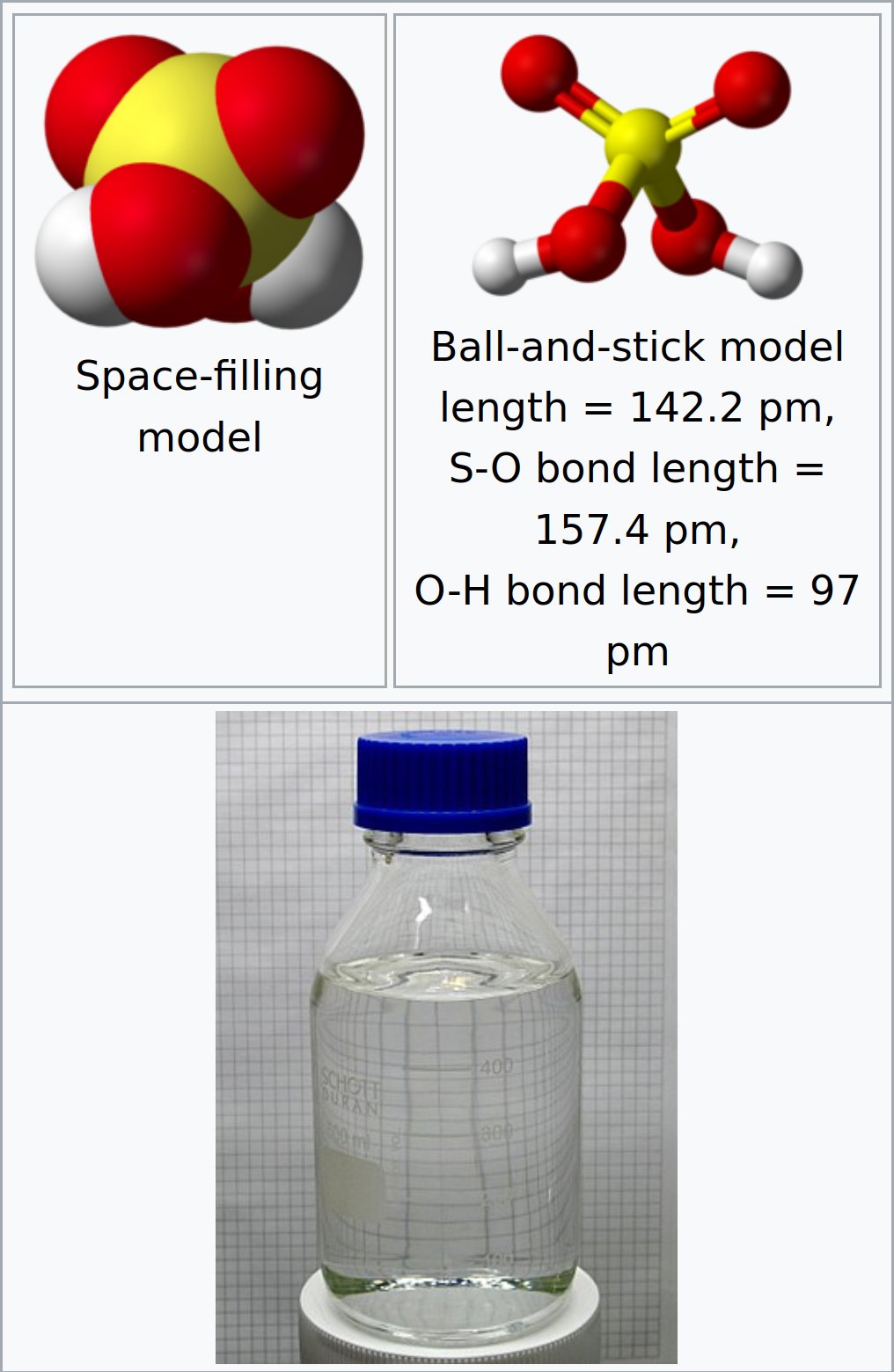
Sulfuric Acid
Sulfuric Acid (American spelling and the preferred IUPAC name) or Sulphuric Acid (Commonwealth spelling), known in antiquity as Oil of Vitriol, is a Mineral Acid composed of the Elements Sulfur, Oxygen and Hydrogen, with the molecular formula H2SO4. It is a colorless, odorless and viscous Liquid that is miscible with Water.
Pure Sulfuric Acid does not occur naturally due to its strong affinity to Water Vapor; it is hygroscopic and readily absorbs Water Vapor from the Air. Concentrated Sulfuric Acid is highly corrosive towards other Materials, from Rocks to Metals, since it is an Oxidant with powerful dehydrating properties. Phosphorus Pentoxide is a notable exception in that it is not dehydrated by Sulfuric Acid, but to the contrary Dehydrates Sulfuric Acid to Sulfur Trioxide. Upon addition of Sulfuric Acid to Water, a considerable amount of heat is released; thus the reverse Procedure of Adding Water to the Acid should not be performed since the heat released may boil the Solution, spraying droplets of hot Acid during the Process.[1]
Diluting Sulfuric Acid
Sulfuric Acid is the most dangerous common Acid to dilute. Partly, this is because it reacts so violently with skin and clothing. Sulfuric Acid quickly dehydrates Proteins and Carbohydrates in skin and muscle. The Acid is much heavier than Water, so Water added to it reacts with the Top Layer first. A lot of heat gets generated, even when Sulfuric Acid and Water are mixed properly. Mixing 100 ml of concentrated Sulfuric Acid and 100 ml of water at 19 °C reaches a temperature over 131 °C (well past the boiling point of Water) in under a minute.
It’s the hydration reaction that generates all that heat:
H2SO4 + H2O → H3O+ + HSO4– [2]
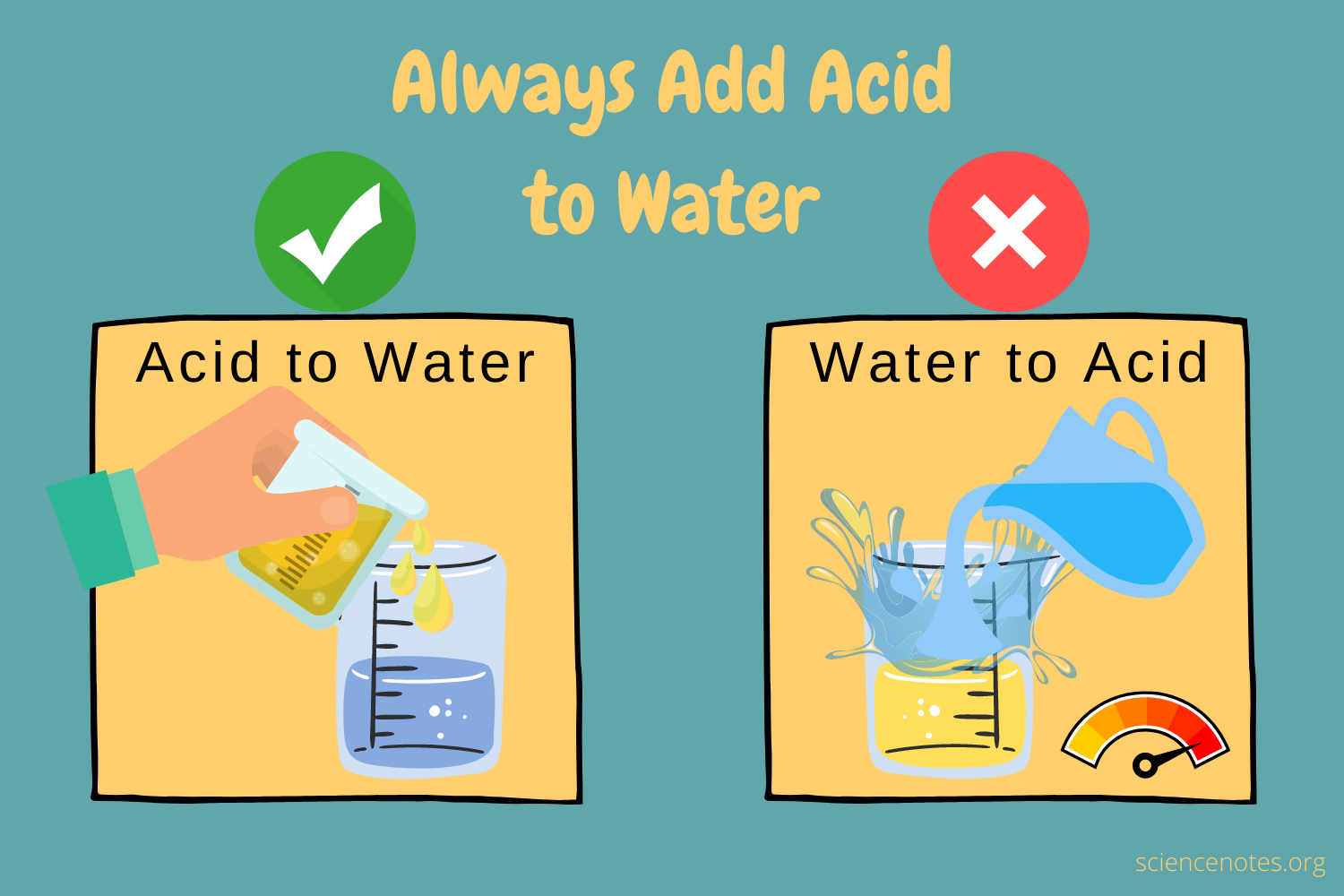
For safety reasons the old saying in Chemistry Labs goes as "Always add Acid to Water, not Water to Acid"
Figure 1 - Always Add Acid to Water[2]
Grades of Sulfuric Acid
Although nearly 100% Sulfuric Acid Solutions can be made, the subsequent loss of SO3 at the boiling point brings the concentration to 98.3% Acid. The 98.3% Grade is more stable in storage, and is the usual form of what is described as "Concentrated Sulfuric Acid". Other concentrations are used for different purposes. Some common concentrations are
Mass fraction
H2SO4 |
Density
(kg/L) |
Concentration
(mol/L) |
Common name |
| <29% |
1.00-1.25 |
<4.2 |
Diluted sulfuric acid |
| 29–32% |
1.25-1.28 |
4.2-5.0 |
Battery Acid (used in
Lead–Acid Batteries) |
| 62–70% |
1.52–1.60 |
9.6–11.5 |
Chamber Acid
Fertilizer Acid |
| 78–80% |
1.70-1.73 |
13.5-14.0 |
Tower Acid
Glover Acid |
| 93.2% |
1.83 |
17.4 |
66 °Bé Acid
("66-degree Baumé") |
| 98.3% |
1.84 |
18.4 |
Concentrated
Sulfuric Acid |
1. Sulfuric Acid, Wikipedia
2. Add Acid to Water or Water to Acid? Safely Diluting Acids, SCIENCE NOTES