Steel slag, a by-product of steel making, is produced during the separation of the molten steel from impurities in steel-making furnaces. The slag occurs as a molten liquid melt and is a complex solution of silicates and oxides that solidifies upon cooling.
Virtually all steel is now made in integrated steel plants using a version of the basic oxygen process or in specialty steel plants (mini-mills) using an electric arc furnace process. The open hearth furnace process is no longer used.
In the basic oxygen process, hot liquid blast furnace metal, scrap, and fluxes, which consist of lime (CaO) and dolomitic lime (CaO.MgO or "dolime"), are charged to a converter (furnace). A lance is lowered into the converter and high-pressure oxygen is injected. The oxygen combines with and removes the impurities in the charge. These impurities consist of carbon as gaseous carbon monoxide, and silicon, manganese, phosphorus and some iron as liquid oxides, which combine with lime and dolime to form the steel slag. At the end of the refining operation, the liquid steel is tapped (poured) into a ladle while the steel slag is retained in the vessel and subsequently tapped into a separate slag pot.
There are many grades of steel that can be produced, and the properties of the steel slag can change significantly with each grade. Grades of steel can be classified as high, medium, and low, depending on the carbon content of the steel. High-grade steels have high carbon content. To reduce the amount of carbon in the steel, greater oxygen levels are required in the steel-making process. This also requires the addition of increased levels of lime and dolime (flux) for the removal of impurities from the steel and increased slag formation.
There are several different types of steel slag produced during the steel-making process. These different types are referred to as furnace or tap slag, raker slag, synthetic or ladle slags, and pit or cleanout slag. Figure 1 presents a diagram of the general flow and production of different slags in a modern steel plant.
The steel slag produced during the primary stage of steel production is referred to as furnace slag or tap slag. This is the major source of steel slag aggregate. After being tapped from the furnace, the molten steel is transferred in a ladle for further refining to remove additional impurities still contained within the steel. This operation is called ladle refining because it is completed within the transfer ladle. During ladle refining, additional steel slags are generated by again adding fluxes to the ladle to melt. These slags are combined with any carryover of furnace slag and assist in absorbing deoxidation products (inclusions), heat insulation, and protection of ladle refractories. The steel slags produced at this stage of steel making are generally referred to as raker and ladle slags.
Figure 1 - Overview of slag production in modern integrated steel plant
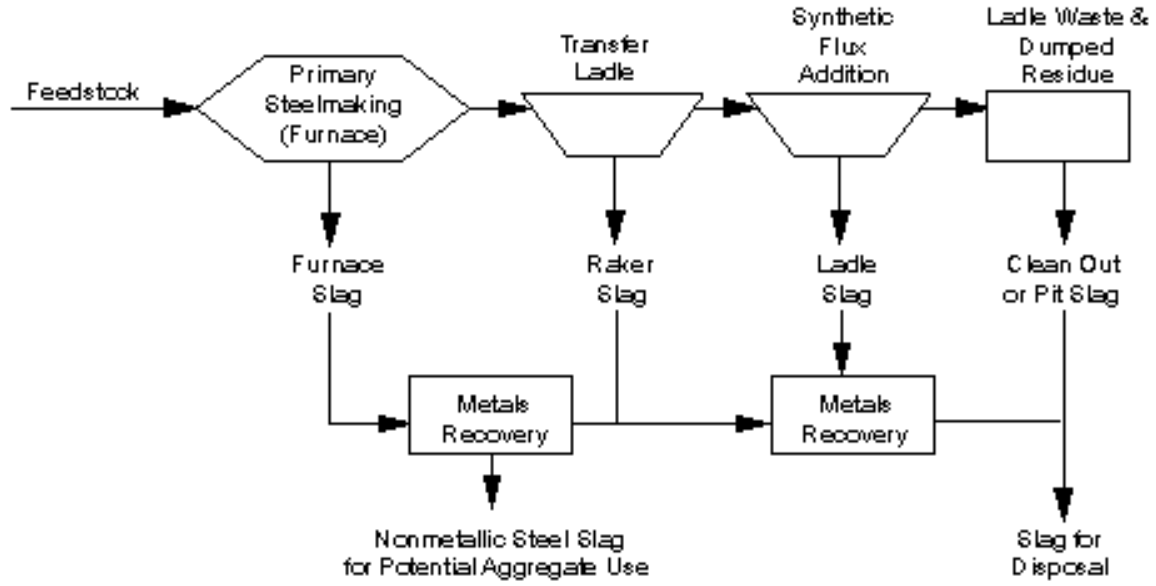
Pit slag and clean out slag are other types of slag commonly found in steel-making operations. They usually consist of the steel slag that falls on the floor of the plant at various stages of operation, or slag that is removed from the ladle after tapping.
Because the ladle refining stage usually involves comparatively high flux additions, the properties of these synthetic slags are quite different from those of the furnace slag and are generally unsuitable for processing as steel slag aggregates. These different slags must be segregated from furnace slag to avoid contamination of the slag aggregate produced.
In addition to slag recovery, the liquid furnace slag and ladle slags are generally processed to recover the ferrous metals. This metals recovery operation (using magnetic separator on conveyor and/or crane electromagnet) is important to the steelmaker as the metals can then be reused within the steel plant as blast furnace feed material for the production of iron.
While most of the furnace slag is recycled for use as an aggregate, excess steel slag from other operations (raker, ladle, clean out, or pit slag) is usually sent to landfills for disposal. The primary applications for steel slag in the United States are its use as a granular base or as an aggregate material in construction applications.
Steel slag aggregates are highly angular in shape and have rough surface texture. They have high bulk specific gravity and moderate water absorption (less than 3 percent).
The chemical composition of slag is usually expressed in terms of simple oxides calculated from elemental analysis determined by x-ray fluorescence. Table 1 lists the range of compounds present in steel slag from a typical base oxygen furnace. Virtually all steel slags fall within these chemical ranges but not all steel slags are suitable as aggregates. Of more importance is the mineralogical form of the slag, which is highly dependent on the rate of slag cooling in the steel-making process.
Table 1 - Typical steel slag chemical composition
| Constituent |
Composition (%) |
| CaO |
40 - 52 |
| SiO2 |
10 - 19 |
| FeO |
10 - 40
(70 - 80% FeO, 20 - 30% Fe2O3) |
| MnO |
5 - 8 |
| MgO |
5 - 10 |
| Al2O3 |
1 - 3 |
| P2O5 |
0.5 - 1 |
| S |
< 0.1 |
| Metallic Fe |
0.5 - 10 |
Source: U.S. Department of Transportation, Federal Highway Administration, User Guidelines for Waste and Byproduct Materials in Pavement Construction.