Product
- Product
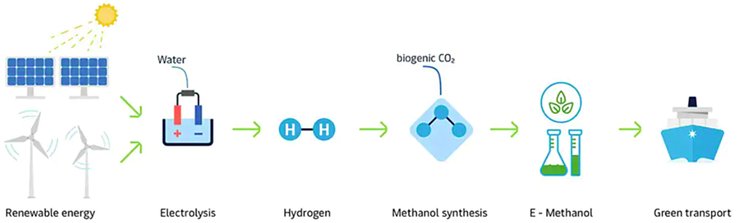
- e-Methanol
- Abbreviation
- eMeOH
- Names
- Green methanol
-

- #PS654
- Main Product
- Methanol
- Segment
- Chemicals
- Main-Family
- Functional Organic Products
- Sub-Family
- Alcohols
- Physical State
-
Liquid
Description
Your insights will be shown here
Product Communicator
| Title | Date | |
|---|---|---|

|
3/19/2025 |
Identifiers
-
 CAS Number
CAS Number
- 67-56-1
-
 EC Number
EC Number
- 200-659-6
-
 ECHA InfoCard
ECHA InfoCard
- 100.000.599
-
 IUPAC Name
IUPAC Name
- Methanol
-
 PubChem ID
PubChem ID
- 887
Chemical Data
- Chemical Formula
-
CH4O
- Molecular Weight (g/mol)
- 32.04
- Boiling Point (°C)
- 64.7
- Melting Point (°C)
- -97.6
- Sulfur Content (wt%)
- 0
- Specific Gravity
- 0.79
Crude Data
- API Gravity
- 47.61
- Country
Product Settings
- Default
- Status
- A
Content provided by
| Transaction | Name | Date |
|---|---|---|
| Modified by |
|
6/4/2025 12:52 PM |
| Added by |
|
3/19/2025 8:10 AM |