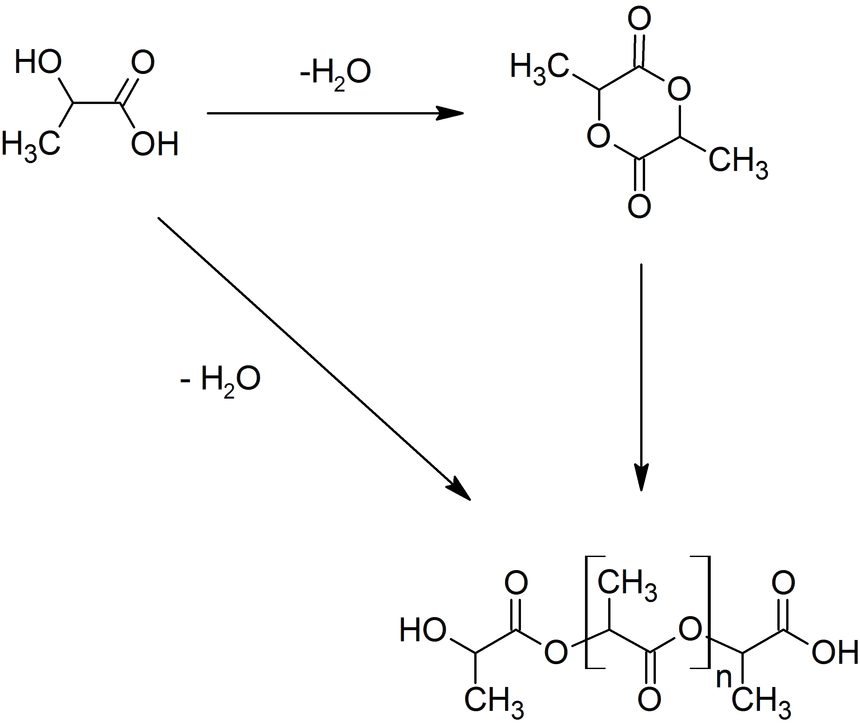Description
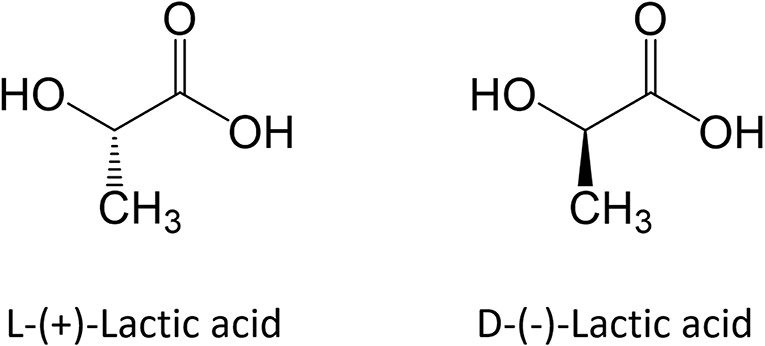
Polylactic acid is a hydrophobic Polymer that belongs to the class of Biomaterials commonly referred as Poly-α-hydroxy Acids, Poly-α-esters or Aliphatic Polyesters. It is synthesized starting from Lactic Acid (LA; 2-Hydroxypropanoic Acid), which a water-soluble Monomer that exhibits two enantiomeric forms, namely L-(+)-LA and D-(-)-LA, as shown in Fig. 1.
Figure 1 - Enantiomeric forms of Lactic Acid
PLA can be produced starting from pure L-Lactic and D-Lactic isomers, which leads to Poly-L-Lactic Acid (PLLA) and Poly-D-Lactic Acid (PDLA) Homopolymers, respectively; if a Racemic Mixture of L- and D-Monomers is employed, Poly-D,L-Lactic Acid (PDLLA) Copolymer is obtained. The stereochemistry has a relevant impact on material properties: PLLA is a semi-crystalline Polymer, while PDLLA is an amorphous Polymer with no melting point. In addition, degradation rate of PLLA is significantly slower than PDLLA, because of the presence of crystalline regions.
PLA is widely employed in the biomedical field because of its biocompatibility and its processability, since it can be processed with a wide range of techniques, such as extrusion, injection molding, injection stretch blow molding, film and sheet casting, extrusion blow film, thermoforming, foaming, fiber spinning, electro spinning, blending, compounding, and nanocompositing.
PLA Manufacturing
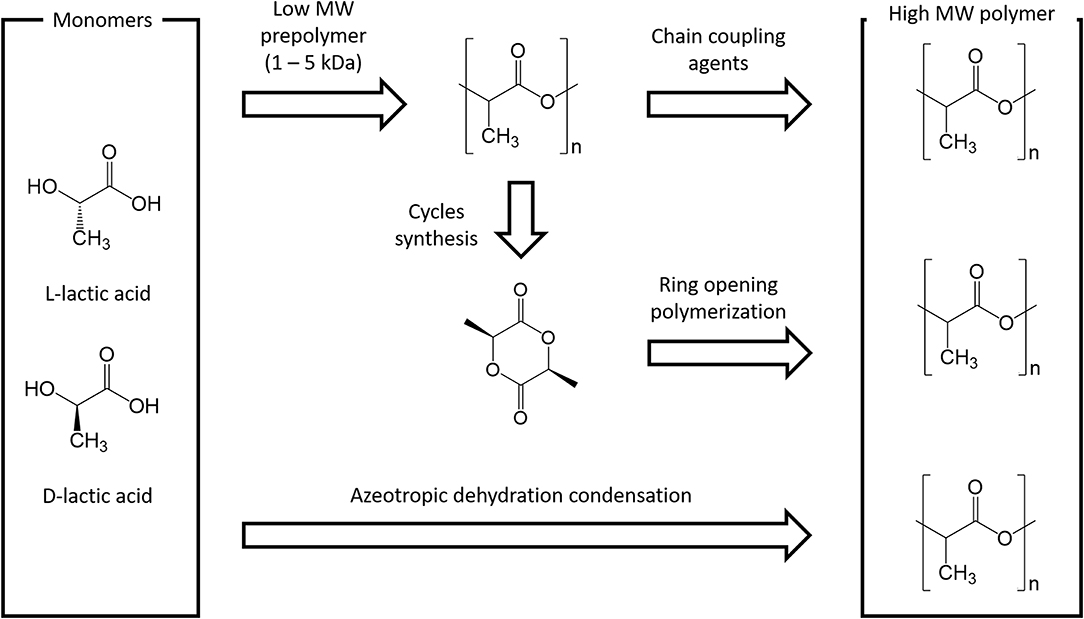
Polymer Synthesis can be carried out through Step Growth Polymerization or Ring Opening Polymerization (ROP) as shown in Fig. 2.
Figure 2 - Main PLA Production Routes
Step Growth Polymerization simply takes advantage of the reactivity of the two LA functional groups: indeed, the Polycondensation of Hydroxyl and Carboxyl Moieties leads to the formation of the Ester bonds that constitute Polymer backbone. This synthetic route has several drawbacks: long residence times are required for longer chains (leading to unwanted Side Reactions, like Transesterification), challenging Reaction conditions (temperatures up to 250°C and vacuum up to 100 mbar) and continuous Water (side product of Polycondensation) removal. Chain Extenders (e.g., Isocyanates or Epoxides) can be in principle employed, although this approach has an inevitable impact on Material purity and quality.
At industrial scale ROP is the most popular Process because of its advantages: mild Process conditions, short residence times, absence of Side Products and high molecular weights. The most widely used Catalyst is 2-Ethylhexanoic Tin(II) Salt (also referred as Stannous Octoate [Sn(Oct)2]), approved by United Stated Food and Drug Administration (FDA) and usually employed along with an Alcohol as Cocatalyst. The real bottleneck of ROP is the availability of Cyclic Monomers as well as their optical and chemical purity, since Impurities have detrimental effects on Material properties due to the sensitivity of the Reaction to residual Non-cyclic Monomers. The cyclic Raw Material for PLA is constituted by Cyclic Dimer Lactide, which exhibits three Stereoisomeric forms, as shown in Fig. 3: LL-, DD-, and D,L-(also referred a Meso-lactide). Lactide is usually produced through backbiting kinetic mechanism is then (promoted with suitable Process conditions) starting from low molecular weight Prepolymer; Cycles are eventually collected by Distillation. Other Synthesis routes are Azeotropic Dehydration and Enzymatic Polymerization.
Figure 3 - Cyclic dimers for ROP process

Physical and Chemical Properties
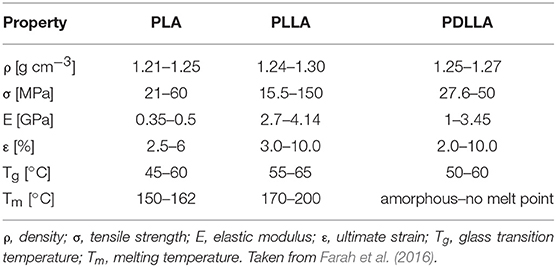
PLA can be seen as a “family” of Polymers, which include Homopolymers PLLA and PDLA (synthesized from pure L- or D-Lactic Acid) and the Copolymer PDLLA (obtained from the Racemic Mixture). This has a remarkable impact on Material properties because of the involved stereochemistry: PLLA and PDLA are semicrystalline Polymers, while PDLLA is usually amorphous. The final crystallinity depends also on the thermal and mechanical history, mainly due to fabrication processes. Mechanical properties are summarized in Table 1; values are expressed as ranges, since they strongly depend on the characteristic of the tested Material (molecular weight, crystallinity, processing, etc.) as well as testing procedure. Polymer crystallinity influences mechanical and physical properties such as hardness, modulus, tensile strength, stiffness, and melting points. If the amount of PLLA is higher than 90% the Polymer is semicrystalline, while lower amounts (and thus a lower optical purity) leads to an Amorphous Polymer. The density values lie in small range depending on the composition.
Table 1 - Mechanical properties of PLA-based Polymers
Another popular way to tune Material properties is the Copolymerization with Glycolic Acid, which leads to the well-known Polylactic-co-glycolic Random Copolymer (PLGA). Copolymerization is also performed with Caprolactone, which allows obtaining Polylactic-co-caprolactone (PLCL). Another strategy to improve Material hydrophilicity is the synthesis of PLA and Polyethylene Glycol (PEG) Block Copolymers
Biodegradation
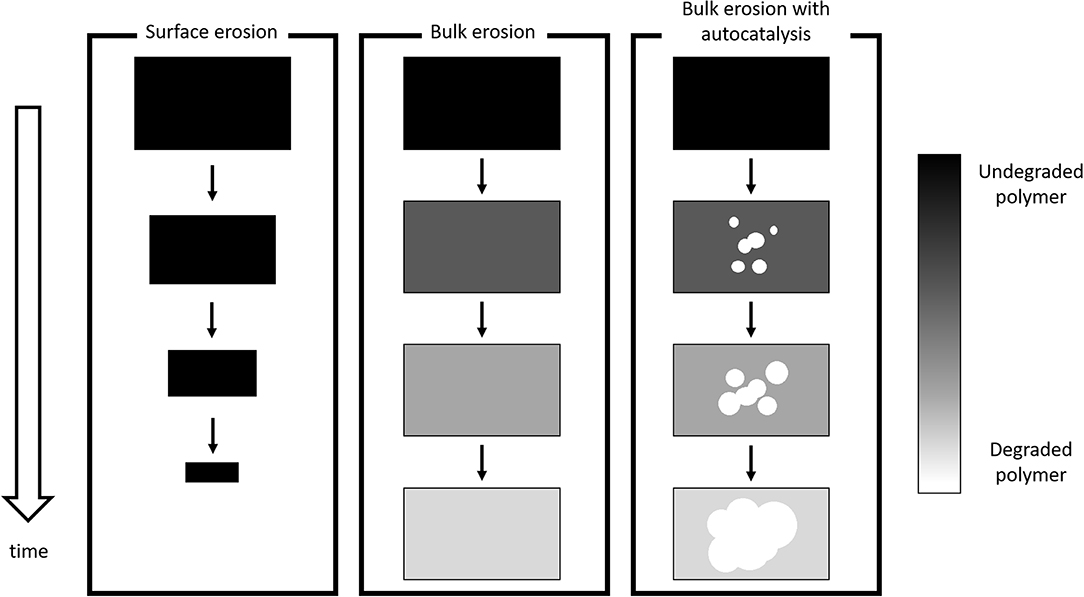
PLA (as well as its Copolymers) degrades because of Hydrolysis Mechanism: Water breaks the Ester Bonds that constitute Polymer Backbone, according to the following mechanism (4):
| |
Pn+m + H2O + H+ ↔ Pn + Pm + H+ |
(4) |
where Pn+m, Pn, and Pm are Polymer Chains composed by n+m, n, and m Monomer Units, respectively, H2O is a Water Molecule and H+ indicates that Hydrolysis is catalyzed in acidic environment. The most important phenomena that govern PLA Degradation are currently rationalized and accepted in scientific literature.
Figure 4 - Schematic of Degradation Mechanisms
References
Casalini T, Rossi F, Castrovinci A and Perale G (2019) A Perspective on Polylactic Acid-Based Polymers Use for Nanoparticles Synthesis and Applications. Front. Bioeng. Biotechnol. 7:259. doi: 10.3389/fbioe.2019.00259