Technology
- Name
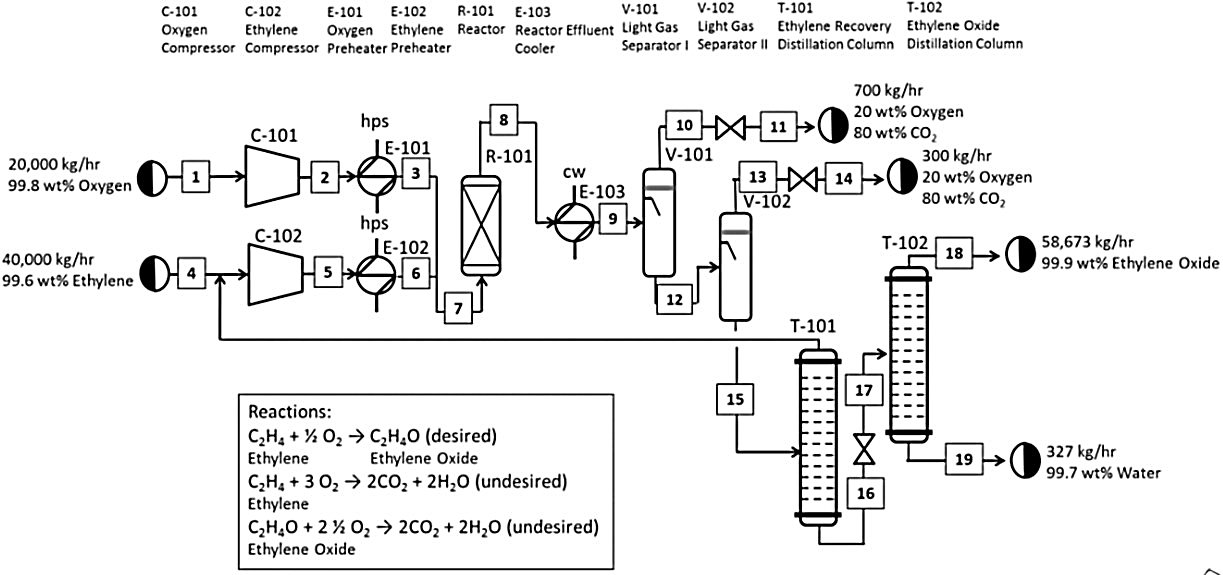
- Generic EO Process
- Owner
-
/ Undefined Technology Provider - Brand
- Generic EO Process
- Process
- Oxidation
- Type
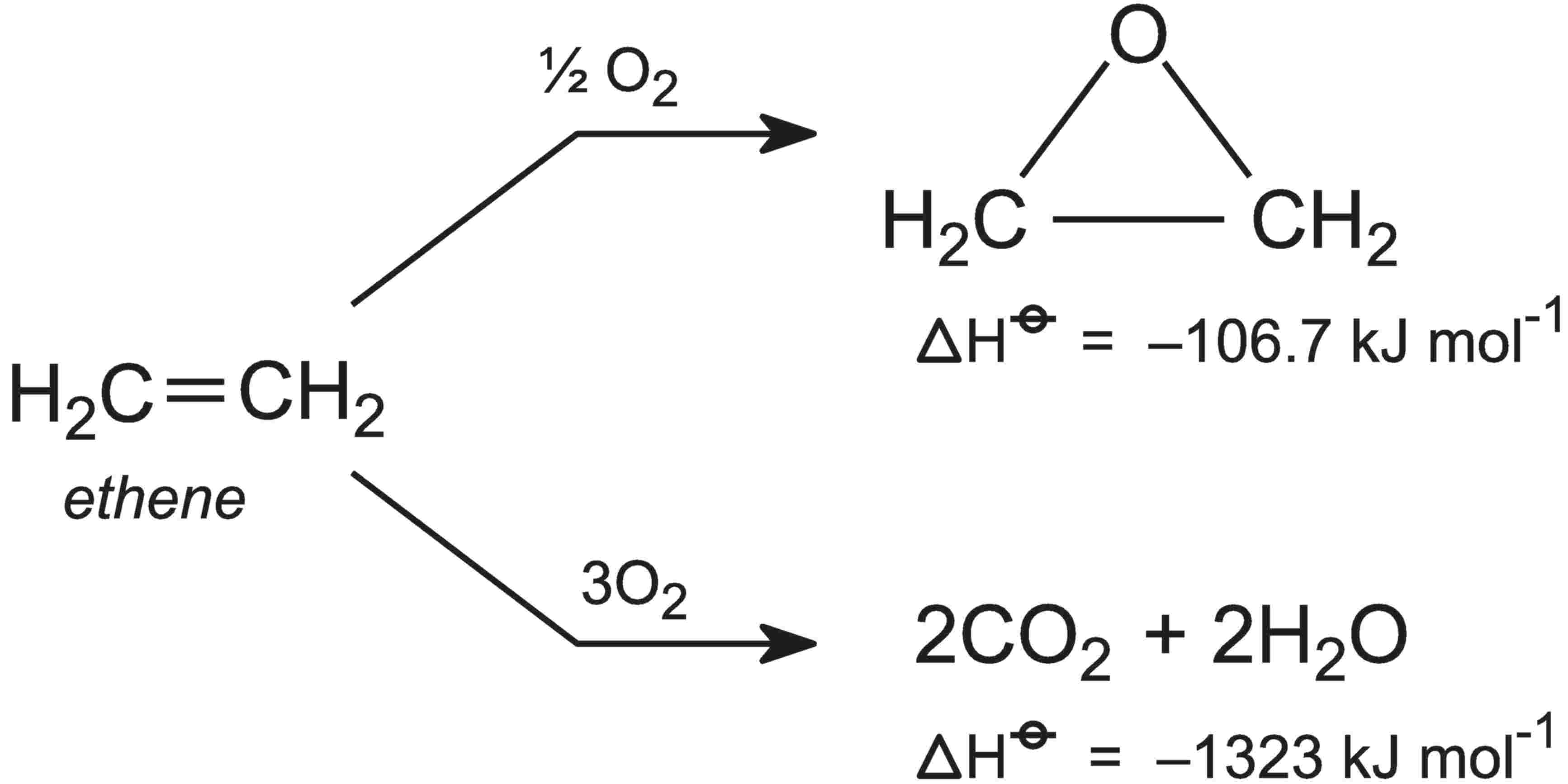
- Ethylene Oxidation into Ethylene Oxide
- Available
-

- #TE258
Description
Your insights will be shown here
| Title | Date |
|---|
| Technology Unit |
|---|
| Compressor |
| Distillation Column |
| Heat Exchanger |
| Light Gas Separator |
| Reactor |
Content provided by
| Transaction | Name | Date |
|---|---|---|
| Modified by |
|
10/12/2024 6:55 AM |
| Added by |
|
10/6/2024 3:32 PM |