Purpose of crude oil dehydration and desalting
Crude oil received after transportation contains dissolved salts trapped in small brine droplets dispersed throughout the oil volume. These droplets are too small for self-extraction and gravity settling. In addition to salts, crude oil contains other oleophobic impurities:
- Salts: Mainly chlorides and sulfates of sodium (Na), potassium (K), calcium (Ca), and magnesium (Mg)
- Sediments: Silt, sand, mud, iron oxide, sulfides, and corrosion products
- Water: Present in soluble, emulsified, and finely dispersed forms
- Organic salts: Heavy amine hydrochloride salts derived from asphaltene fractions
If crude oil is left untreated before processing, these contaminants can cause severe operating failures and maintenance problems, including corrosion, fouling, catalyst poisoning, and equipment damage. Crude oil dehydration and desalting is a critical initial processing step in refineres:
- Crude oil dehydration removes water present in the crude oil
- Desalting removes dissolved salts by extracting them from the oil phase into a water phase
- Since inorganic salts are dissolved in the brine droplets, effective water removal also achieves desalting
The process also removes a considerable percentage of suspended solids (sand, clay, soil particles, and corrosion products from pipelines and upstream equipment).
Fundamental Mechanism: Salt Extraction, Not Mere Demulsification
Refinery desalting is fundamentally a salt extraction and dilution process, not simply a demulsification process. The key distinction is critical to understanding the chemistry and chemical selection.
- Fresh Water Injection
Fresh water, typically 4-8% of the crude charge volume, is injected and intimately mixed with the incoming crude oil. This fresh water must be emulsified into fine droplets to maximize contact with existing brine droplets. The fresh water acts as a diluent to reduce salt concentration in the aqueous phase.
- Specialized Desalter Demulsifiers
A polymeric surfactant, specifically formulated as a "desalter demulsifier" or "salt extraction agent," is added to the system. This chemical creates a fine but unstable water-in-oil emulsion. This is distinctly different from conventional demulsifiers that simply destabilize existing emulsions.
The desalter demulsifier works by displacing natural emulsion stabilizers (asphaltenes, resins, and finely divided solids) from the oil-water interface, weakening the protective films around droplets. If an ordinary demulsifier that simply destabilized emulsions were used, it would not create the fine emulsion necessary for effective salt extraction and dilution.
- Salt Dilution Mechanism
The fresh water emulsion droplets coalesce with the existing brine droplets containing concentrated salts. This coalescence dilutes the salt concentration approximately 10-fold (typically from 0.5% to 5.0% salt in water). The combination of fresh water injection and coalescence is essential to achieve the dramatic reduction in salt content.
- Critical Droplet Size Separation
Above a critical droplet size, the larger coalesced water droplets settle faster than the upward oil velocity and collect in the lower water phase, which is continuously withdrawn. Below this critical size, some droplets remain entrained in the oil phase but carry significantly reduced salt content due to the dilution effect. The settling rate depends on the square of the droplet diameter, making coalescence a key factor in separation efficiency.
- Solids Water-Wetting and Removal
The desalter demulsifier also converts oil-wet solids to water-wet solids. These water-wetted particles then preferentially migrate to the water phase and settle out with the brine, removing them from the crude oil. This mechanism is critical for removing sand, clay, mud, iron oxide, and corrosion products from the oil stream.
Organic Amine Hydrochloride Salts
In addition to inorganic salts, crude oil can contain organic heavy amine hydrochloride salts derived from the asphaltene fraction. These compounds possess basic nitrogen functionalities in the asphaltene structure that form amine hydrochlorides. Unlike inorganic salts:
- They are not water-soluble and thus cannot be extracted by the water washing process
- They thermally decompose (pyrolyze) during atmospheric and vacuum distillation to release highly corrosive HCl gas
- They represent a persistent corrosion threat that requires downstream corrosion management strategies
This organic salt issue is particularly significant in crudes with high asphaltene content and represents a separate corrosion concern from inorganic salt content. Effective management requires additional corrosion control measures beyond desalting, including the use of corrosion inhibitors in the distillation section.
Methods of Dehydration and Desalting of Crude Oil
Water and oil separate by virtue of their different densities, with gravity as the driving force. Large density differences between oil (ρo) and water (ρw) and low oil viscosities favor easy separation; therefore, light crudes separate faster than heavy crudes. Temperature increase reduces viscosity, so heat application accelerates separation of heavy crudes.
The settling rate depends on the square of the droplet diameter. Drop-to-drop coalescence increases droplet diameter and is therefore a key factor in successful dehydration. However, many oilfield dispersions resist coalescence and are known as "stable dispersions" or "stable emulsions." Oil and water mixtures would be highly unstable except for naturally occurring surfactants and finely divided particles absorbed at oil/water interfaces to form rigid films that resist coalescence. These surfactants stabilize fine droplets which accumulate to form emulsions. Water-in-oil emulsions are termed "normal"; most oilfield emulsions are in this category. Oil-in-water emulsions are termed "reverse."
Three Main Desalting Methods
- Mechanical (Gravity Settling)
Heated crude mixed with wash water is held in a large vessel for gravity settling of water droplets. This method is ineffective as it requires extended residence time for adequate water separation. Separation times can range from hours to days, making this approach impractical for modern refinery throughputs.
- Chemical Desalting
Fresh water and specialized desalter demulsifiers (polymeric surfactants) are injected and intimately mixed with the crude oil. The demulsifier creates a fine but unstable emulsion that facilitates salt extraction through fresh water-brine coalescence and dilution. The process achieves faster separation than mechanical methods but still relies primarily on gravity settling.
Figure 1 — A simplified chemical desalting process
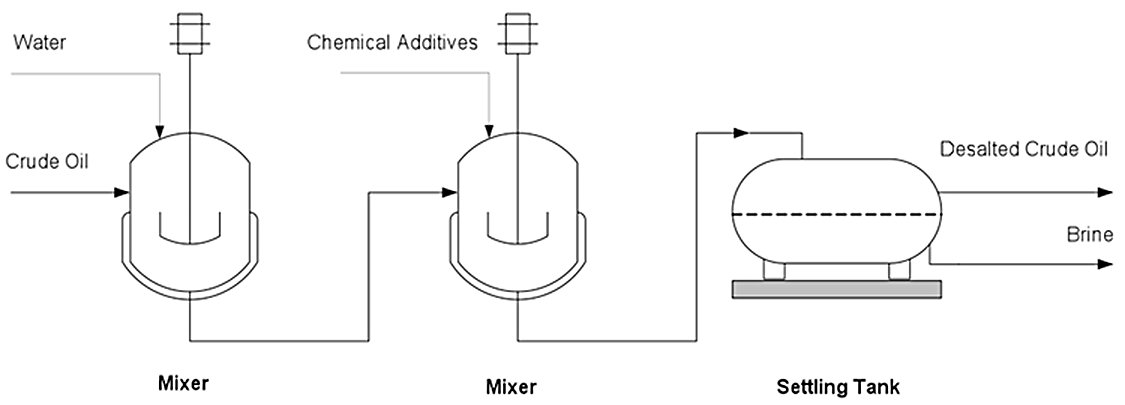
Chemical desalting typically employs one-stage or two-stage vessel configurations depending on the required salt removal efficiency:
- One-stage configuration: Achieves approximately 90% salt removal. Suitable for crudes with moderate initial salt content.
- Two-stage configuration: Achieves up to 99% salt removal. Required for high-salt crudes or when very low product salt specifications (< 1.5 PTB) must be met.
|
In the two-stage configuration, demulsifier injection occurs before the mixing valve in both stages. Fresh water is fed to the second stage, and effluent water from the second stage is recycled to the first stage. This counter-current water flow maximizes salt extraction efficiency while minimizing fresh water consumption.
- Electrical Desalting
An electrostatic field is applied by high-voltage electrodes within the desalting vessel. The electrostatic field induces polarization on water droplets, creating attractive forces that promote coalescence and accelerate droplet growth. The enlarged droplets settle faster under gravity. The separated water is continuously withdrawn from the bottom of the vessel, leaving desalted and dehydrated crude oil.
Electrical desalting is the most effective method and is the standard technology in modern refineries. Typical operating parameters include:
- Voltage: 3,000 to 10,000 V/cm electric field strength
- Temperature: 120-150°C for optimal viscosity reduction and coalescence
- Residence time: 20-45 minutes
- Fresh water addition: 4-8% of crude charge volume
- Target salt removal: 90-99% depending on configuration
|
Figure 2 — A general process flow diagram for one- and two-step desalting processses

Dehydration and Desalting Equipment
Various vessel types are employed for crude oil dehydration and desalting, each suited to different crude characteristics and process stages:
| Equipment |
Application |
Comments |
| Free water knockout (FWKO) separator |
High water cut crudes where bulk water separates quickly |
Final crude polishing to export quality requires other methods |
| Dehydration separator |
Low water crudes requiring dehydration to about 1-5% water |
Usually located downstream of FWKO separators in offshore applications |
| Heater treater |
Difficult emulsions or very viscous crudes |
Choice based on economic arguments; can operate above 100°C |
| Wash tank |
General purpose, particularly useful with higher water cut crudes |
Requires careful design of internals to avoid channelling |
| Concentric wash tank |
Heavy, high water cut crudes |
More expensive than conventional designs and more difficult to operate |
| Settling tank |
General purpose where ample tankage is available |
Poor choice for high water cut crudes |
| Electrostatic coalescer |
Deep dehydration required (to about 0.5% water) |
More sophisticated with greater potential for problems; short-circuiting risks. |
Typical Plant Configuration
Depending on desired salt content in desalted crude oil, one-stage or two-stage desalting processes are applied. For refining purposes, a maximum salt concentration of 1.5 PTB (pounds of salt measured as NaCl per thousand barrels) is typically specified.
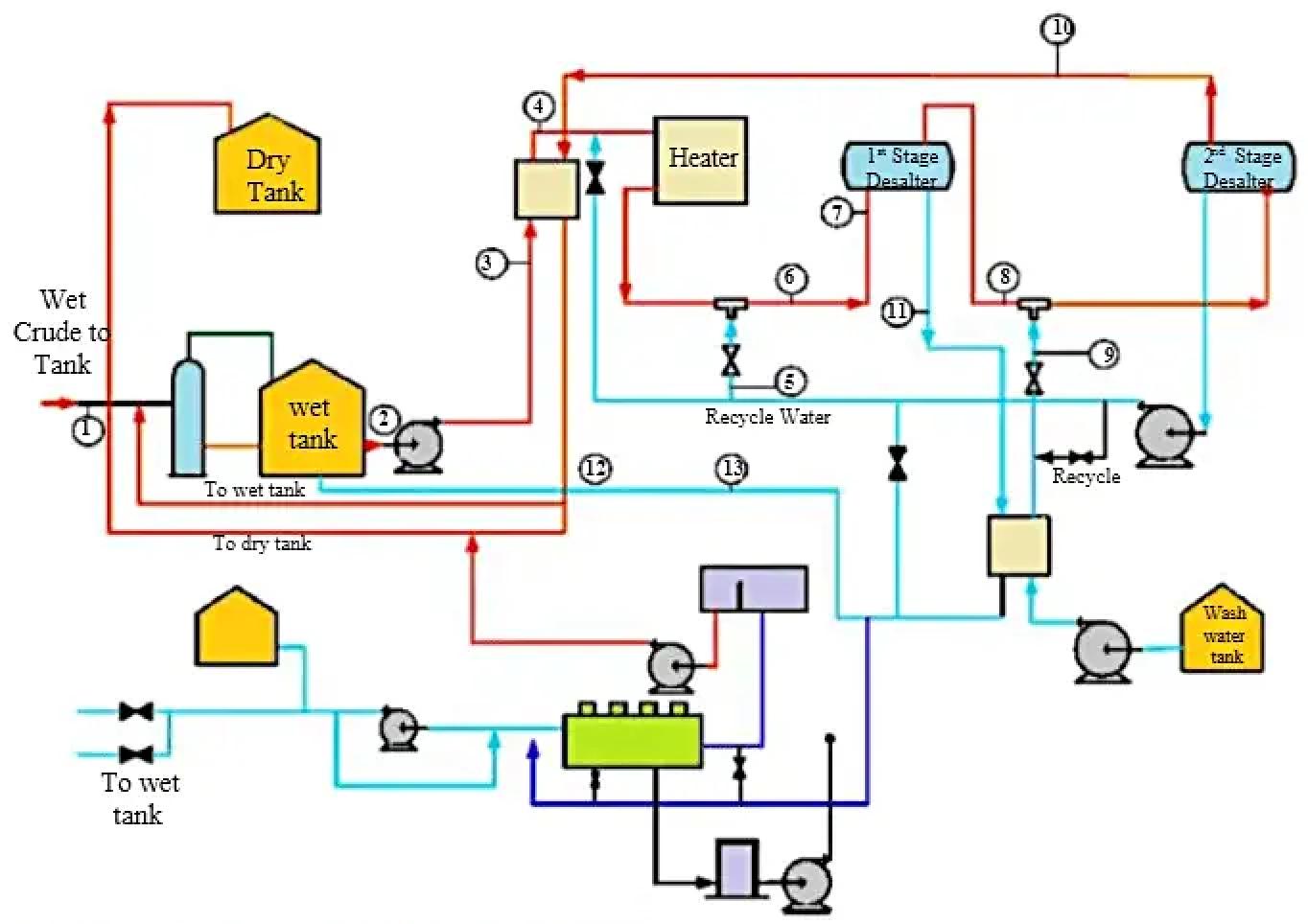
Figure 3 — A typical two-step crude desalting process flow scheme
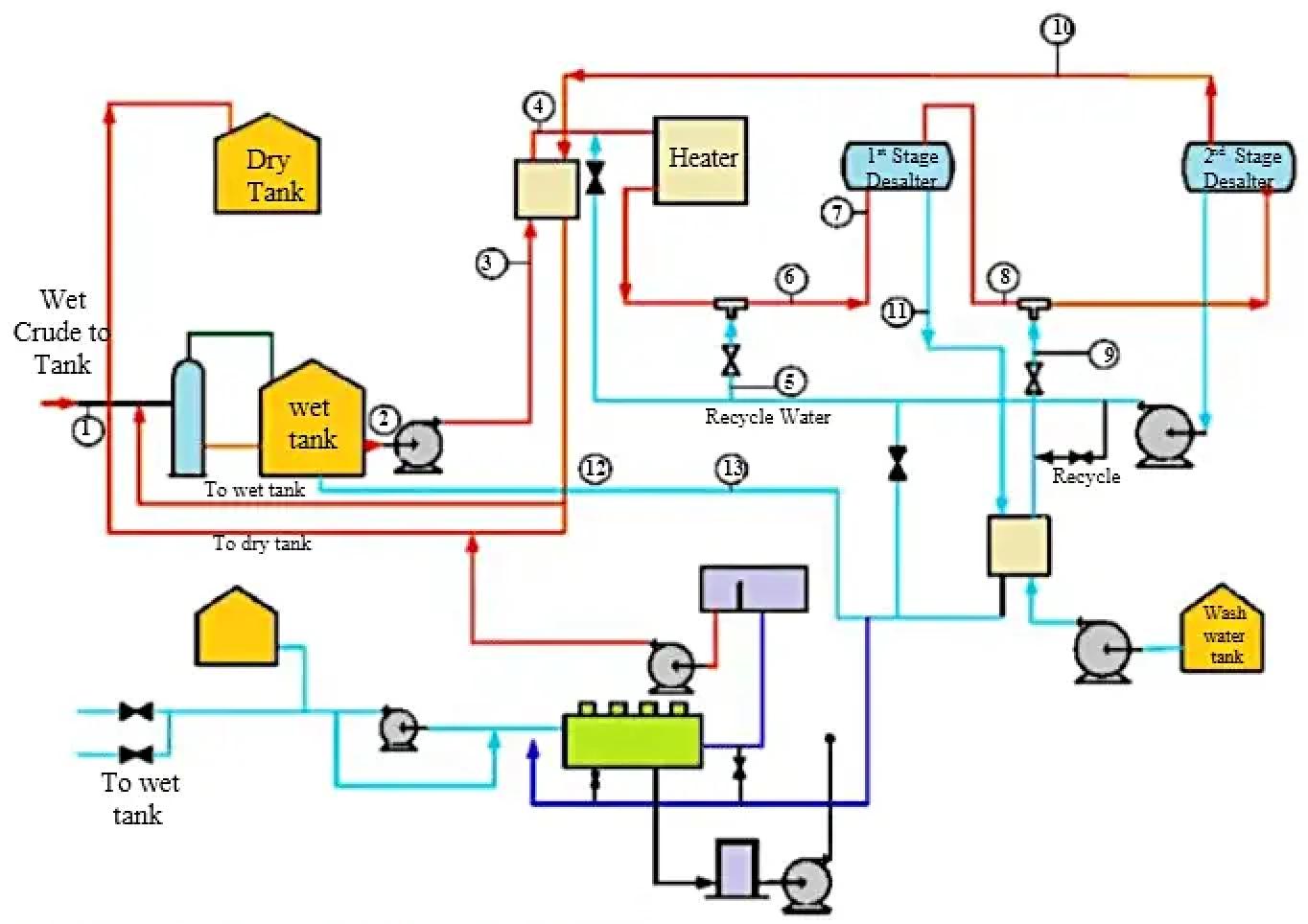
Two-Stage Desalting Process Flow
A typical two-stage electrostatic desalting configuration includes the following unit operations:
- Wet crude storage tank: Receiving point for transported crude oil with high water and salt content
- First-stage demulsifier injection: Addition of desalter demulsifier before mixing valve
- Heat exchange: Preheating of crude oil against desalted crude oil outlet (heat recovery)
- Fired heater: Temperature control to 120-150°C for optimal separation
- Wash water recycle from second stage: Counter-current water feeding from downstream separator
- First-stage mixing valve: Creates fine emulsion through turbulent mixing and shear
- First-stage electrostatic desalter vessel: Initial salt extraction and water-solid removal
- Intermediate crude flow: Partially desalted crude to second stage
- Second-stage demulsifier injection: Additional demulsifier injection before second mixing valve
- Fresh wash water feed: Demineralized water from wash water tank and oil-water heat exchanger
- Second-stage mixing valve: Final fine emulsion creation
- Second-stage electrostatic desalter vessel: Final salt extraction to specification
- Desalted crude oil outlet: Product to atmospheric distillation unit at target salt/water content
- Brine recycle loop: Second-stage water returned to first stage (counter-current operation)
- First-stage brine withdrawal: Spent brine to water treatment plant and/or disposal
- BS&W analyzer with diverting valve: Real-time quality control and batch routing
- Formation water removal: Free water settled in wet crude tank withdrawn to water treatment
Process Variables and Performance Factors
Key variables affecting desalting efficiency include:
- Wash water percentage: Typically 4-8% of crude charge (higher for high-salt crudes)
- Mixing intensity: Sufficient to create fine emulsion without creating overly stable emulsion
- Temperature: Typically 120-150°C depending on crude viscosity and emulsion stability
- Residence time: Typically 20-45 minutes in electrostatic vessels
- Electrostatic field strength: Typically 3,000-10,000 V/cm for effective coalescence
- Demulsifier type, dosage, and injection location: Selection based on crude emulsion characteristics
- Crude oil properties: Density, viscosity, asphaltene content, and natural emulsion stability
- Initial salt and water content: Higher initial levels require two-stage processing or higher chemical dosages
Proper optimization of these variables is essential to achieve target desalting performance while minimizing water and chemical consumption and avoiding operational problems such as excessive emulsion carryover or electrode short-circuiting.
References
Adapted from:
- Pereira J. et al. (2015). Crude Oil Desalting Process. In: Advances in Petrochemicals (Chapter 4). IntechOpen Publisher
- Eser, S., & Riazi, M. R. (2013). ASTM MLN5820131211705 - Crude Oil Refining Processes. In: Petroleum Refining and Natural Gas Processing. pp. 101-125. DOI: 10.1520/MNL5820131211705
- Merchant, P. Jr. and Lacy, S.M. US Patent 4,737,265A: Water Based Demulsifier Formulation and Process for Its Use in Desalting Crude Oil. Jan 23, 1986: Application filed by Exxon Research and Engineering Co
- Dey, A.K. What is Piping Blog. DeSalting and Dehydration of Crude Oil
- Raj, V. All About Piping Blog. Crude Oil Desalter: Purpose, Working, Types, Parts, variables
- Hart, P. Linkedin comment (Dec 2025)