Methanol Synthesis Reaction
Methanol is manufactured using syngas with a H2/CO ratio of 2:1, via CO hydrogenation. The methanol synthesis involves reacting CO, H2 and steam in the presence of copper–zinc oxide catalysts and small amounts of CO2. The reaction path for methanol synthesis (3) follows the water–gas-shift (1) and the hydrogenation of carbon dioxide (2) reactions.
| CO + H2O ↔ H2 + CO2 |
ΔH°R = −41.5 kJ·mol-1 |
(1) |
| CO2 + 3 H2 ↔ CH3OH + H2O |
ΔH°R = -49.7 kJ.mol-1 |
(2) |
| CO + 2 H2 ↔ CH3OH |
ΔH°R = -90.6 kJ·mol-1 |
(3) |
Methanol synthesis is a very exothermic reaction and the conversion is equilibrium limited. The equilibrium constant for methanol synthesis can be calculated by Eq. (4).
| log(Keq) = -7.492.log(T/K) +3971(K/T)-1 +9.218 +1.77×10−3T/K -3.11×10−8(T/K)2 |
(4) |
Reaction Conditions and Catalysis
The production of methanol from syn-gas on an industrial scale using high pressures (250-350 atm) and temperatures (300-400 °C) was first introduced by BASF during the 1920s. From then until the end of World War II, most methanol was produced from coal-derived syn-gas and off-gases from industrial facilities such as coke ovens and steel factories. The use of such feedstocks containing high levels of impurities, was made possible by the design of a catalyst system consisting of zinc oxide and chromium oxide, which was highly stable to sulfur and chlorine compounds.
After World War II, the feedstock for methanol synthesis shifted rapidly to natural gas, which became widely available at very low costs, particularly in the United States. Whereas 71% of the methanol in the United States was still derived from coal in 1946, by 1948 almost 77% was obtained from natural gas. Natural gas is still the preferred feedstock for methanol production because it offers, besides a high hydrogen content, the lowest energy consumption, capital investment and operating costs. Furthermore, natural gas contains fewer impurities such as sulfur and halogenated compounds or metals, which can poison the needed catalysts. When present, these impurities (mostly sulfur in the form of H2S, COS or mercaptans) can, however, also be removed relatively easily. Lower levels of impurities in the syn-gas allowed the use of more active catalysts, operating under milder conditions. This led, during the 1960s, to the development by ICI (later on Synetix, subsequently sold to Johnson Matthey) of a process using a copper-zinc-based catalyst allowing the conversion of syn-gas to methanol at pressures of 50-100 atm and temperatures of 200-300 °C. This low-pressure route is the basis for most current processes for methanol production.
The formation of byproducts (dimethyl ether, higher alcohols, methane, etc.) associated with the old high-pressure technology was also drastically reduced or even eliminated. Production using the high-pressure process is no longer economical, and the last plant based on this technology closed in the 1980s.
All present methanol processes use copper-based catalysts which are extremely active and selective. The reactions take place in a catalytic (mixtures of Co, ZnO, Al2O3 and Mg) reactor and due to the equilibrium limitation, the conversion per pass is typically limited and in the order of 10 %.
Syngas Composition
The stoichiometric number of the syngas feed for methanol synthesis, defined as (H2 − CO2)/(CO + CO2), should be equal or slightly higher than 2.0 to achieve optimum yield of methanol. In fact, a small quantity of CO2 (3–5%) is present in the conventional synthesis of methanol. For this reason, only modifications of traditional copper–zinc oxide (Cu/ZnO) catalysts have been proposed to improve the catalytic activity since these catalysts are also active for the water gas shift reaction in Eq. (2). It is therefore possible to produce methanol from CO2. However, increasing the percentage of CO2 in the syngas affects negatively the cost of the process since part of the H2 is lost to form water.
Asides from the methanol synthesis step, the most crucial part of present methanol plants is the syn-gas generation and purification system, which will depend on the nature and purity of the feedstock used. Although natural gas is generally the preferred feedstock due to the simplicity of obtaining an adequate syngas with low levels of impurities, other routes are also used under given circumstances. Regions rich in coal or heavy oils, but having limited natural gas sources, in view of the high natural gas prices, can turn to these resources to produce methanol despite higher cost for the needed syn-gas purification system.
In practice the composition of the synthesis gas employed for methanol synthesis contains a H2:CO ratio that is larger than 2, partly to enable conversion of CO2 in the syngas and partly to suppress side reactions.
Methanol Production Technology
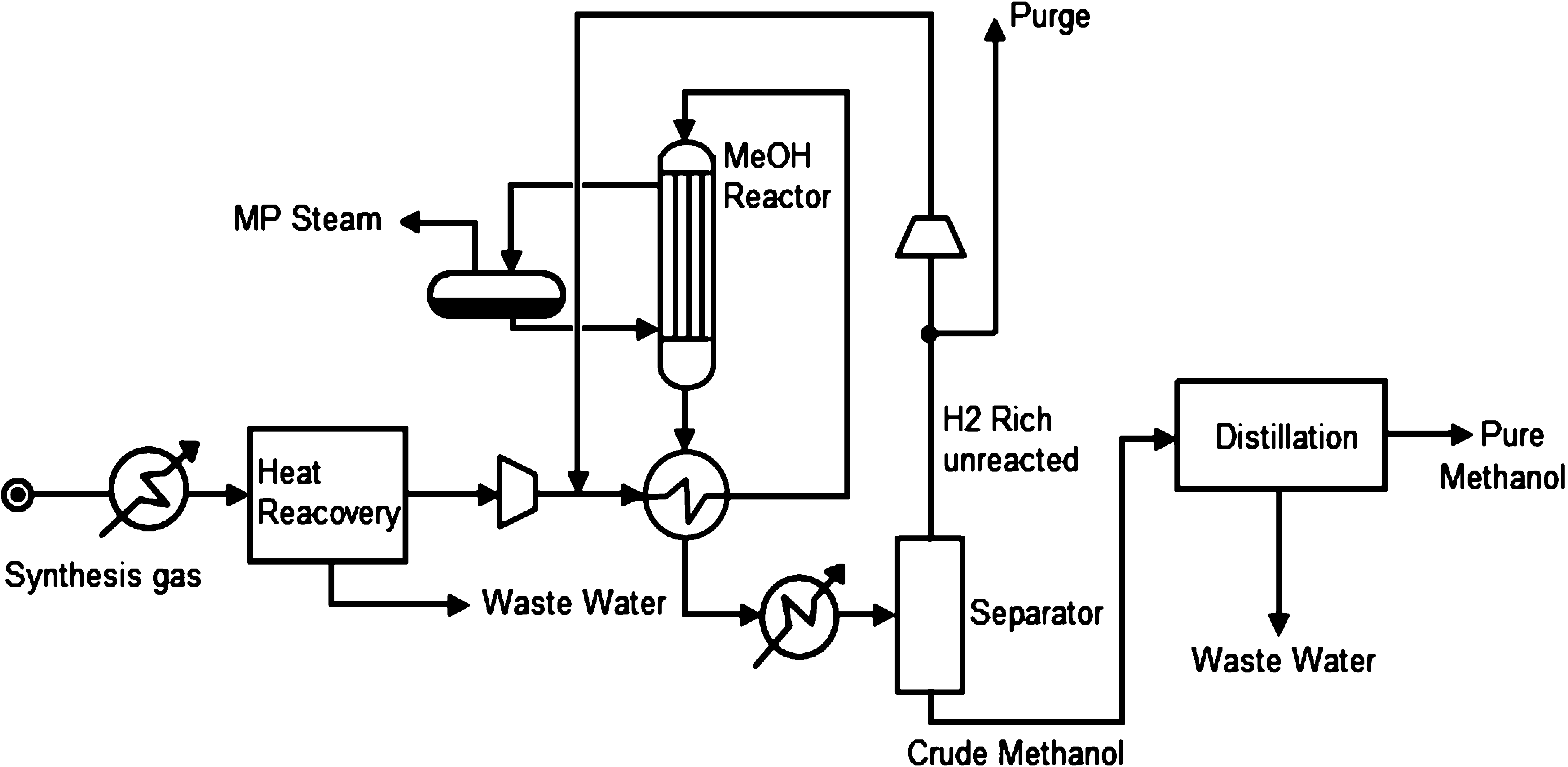
The commercial methanol synthesis reactors can be categorized into gaseous phase and liquid phase reactors.
Modern large-capacity methanol processes are almost exclusively gas-phase processes using low-pressure synthesis loops with copper-based catalysts. Quench-type, multibed intercooled, or isothermal reactors are used to minimize reactor size and maximize recovery of process waste heat. The synthesis loop pressure, reactor type used, and method of waste-heat recovery broadly differentiate gas-phase methanol-synthesis schemes.
In one pass over the catalyst only a part of the syn-gas is converted to methanol. Lighter components are typically separated from reactor product by condensation in a topping column at the reactor's outlet and the remaining syn-gas is recycled to the reactor. The remaining crude methanol contains, however, water and small amounts of other impurities, the distribution and composition of which will depend on the gas feed, reaction conditions, and type and lifetime of the catalyst. Impurities that may be present in methanol include dissolved gases (methane, CO, CO2), dimethyl ether, methyl formate, acetone, higher alcohols (ethanol, propanol, butanol) and long-chain hydrocarbons.
Commercially, methanol is available in three grades of purity: fuel grade, "A" grade, generally used as a solvent, and "AA" or chemical grade. Chemical grade has the highest purity with a methanol content exceeding 99.85% and is the standard generally observed by the industry for methanol production. Depending on the amount of impurities and purity desired, methanol will have therefore to be purified by distillation systems. A two or three column distillation scheme is typically used. The two-column distillation scheme offers low capital expenditures, and the three-column distillation scheme offers low-energy-consumption features. The scheme that integrates better with the syngas preparation and synthesis section is normally selected.
Generally, modern methanol plants have selectivities to methanol higher than 99%, with energy efficiencies above 70%.
Methanol from Syngas Technologies Global Landscape
The following list highlights dominant methanol-from-syngas technology providers as of 2025:
| Provider |
Technology Brand & Highlights |
| Topsoe |
SynCOR Methanol™ (and conventional gas/coal-based processes). Renowned for high single-train capacities (up to 7,200 tpd), oxygen-fired autothermal reforming, and robust copper-zinc catalyst technology. Emphasis on energy integration and low emissions |
| Johnson Matthey |
DAVY™ Methanol, known for versatile loop designs (tube-cooled, steam-raising), optimal pressure/temperature profiles, and proprietary KATALCO™ catalysts. Flexible for all syngas sources (natural gas, coal, biomass), supports very large plants, and continually enhanced sustainability features |
| Air Liquide |
Lurgi MegaMethanol™ for world-scale plants, strong in both natural gas and coal-to-methanol applications. Features robust Lurgi multipurpose technology, advanced distillation, and integrated heat recovery. Modular “MegaMethanol” units now reach beyond 5,000 tpd |
| Casale SA |
Low-Energy Methanol (LEM™) and revamp options. Specializes in compact, modular synthesis loop and revamped legacy plants. Known for process efficiency, reduced steam consumption, and proprietary reactor internals that enhance conversion and catalyst life |
| thyssenkrupp Uhde |
Uhde Methanol technology, notable for flexible feedstock compatibility and advanced radial flow reactors. Strong in large-scale and integration with complex petrochemical platforms |
| Toyo Engineering |
TEC Methanol Process, emphasizes reliability, tailored solutions, and global track record. Integration of proprietary reactors and heat management, and offers EPC and project management worldwide |
References
- ScienceDirect, Methanol Synthesis and references therein. (Accessed 30th Nov 2024)
- GLOBAL SYNGAS TECHNOLOGY COUNCIL, Methanol.
- Brian Williams, 5th Aug 2023, Methanol Economy, Production via Syn Gas.