Technology Type
- Type
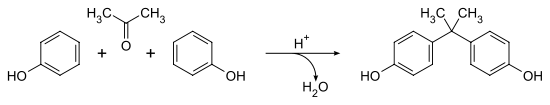
- Condensation of Phenol with Acetone
- Process
- Condensation Reaction
- Abbreviation
- BPA
-

- #TT91
Description
Your insights will be shown here
Image
| Technology | Owner | Entity |
|---|---|---|

|
Badger |
Content provided by
| Transaction | Name | Date |
|---|---|---|
| Modified by |
|
6/17/2025 3:16 PM |
| Added | 11/29/2022 6:40 PM |



.jpeg)





