Product
- Product
- Heating Value
- Names
- Energy Value; Calorific Value
-

- #PS657
- Main Product
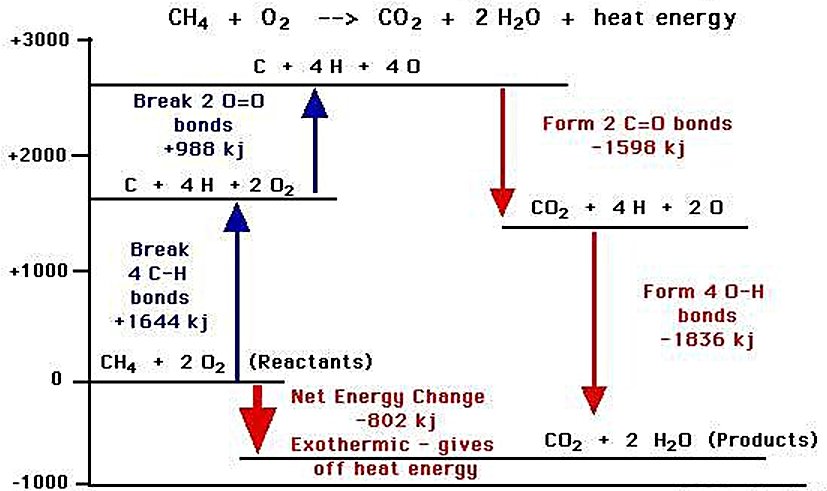
- Combustion Energy Potential
- Segment
- Utilities
- Main-Family
- Potential Energy
- Sub-Family
- Chemical Potential Energy
- Physical State
-
Gas
Description
Your insights will be shown here
Product Communicator
| Title | Date |
|---|
Identifiers
No Identifiers defined
Chemical Data
- Specific Gravity
- 1.00
Crude Data
- API Gravity
- 10
- Country
Product Settings
- Default
- Status
- A
Content provided by
| Transaction | Name | Date |
|---|---|---|
| Modified by |
|
4/2/2025 10:46 AM |
| Added by |
|
3/30/2025 2:33 PM |