Ethylene Chlorination Routes for EDC Production
Ethylene Dichloride production routes are primarily based on:
Thermal cracking of dry, pure EDC then produces Vinyl Chloride Monomer (VCM) and HCl.
When all the HCl generated in EDC cracking is re-used in an oxychlorination section, and when no EDC or HCI is imported or exported, then the VCM unit is called a ‘balanced unit’ (Fig. 1)[1].
Figure 1 - Balanced VCM Unit - Simplified Block Flow Diagram[1].
.jpeg)
Balanced EDC Production Units
By using both, direct chlorination and oxychlorination for EDC production (Fig. 2), balanced units achieve a high level of chlorine utilisation without producing HCl as a by-product.
Assuming a complete incorporation of chlorine input into EDC within a balanced unit, half of the produced EDC originates from each of the applied processes, direct chlorination and oxychlorination.
Additionally, the heat gain from both highly exothermic chlorination processes may be used in the associated VCM production, optimising the overall energy demand of the EDC/VCM/PVC production[1].
Figure 2 - Combined EDC Production Routes[2].
.jpg)
Ethylene Chlorination Reactions
The reactions are represented by the formulae[1]:
| • Direct Chlorination: |
C2H4 + Cl2 → C2H4Cl2 |
ΔHR,0 = −220 kJ/mol |
| • Oxychlorination: |
C2H4 + ½ O2 + 2 HCl → C2H4Cl2 + H2O |
ΔHR,0 = −320 kJ/mol |
| • Cracking: |
C2H4Cl2 → C2H3Cl + HCl |
ΔHR,0 = + 97 kJ/mol |
| • Overall Reaction: |
2 C2H4 + Cl2 + ½ O2 → 2 C2H3Cl + H2O |
|
Raw Materials for EDC Production
For the production of EDC in a balanced unit the raw material requirements comprise ethylene and chlorine, which are generally supplied by pipeline from nearby production facilities.
Chlorine and EDC production sites are often found in close proximity in order to reduce chlorine transportation distances to the EDC process, the single largest chlorine consumer[1].
Direct Chlorination of Ethylene
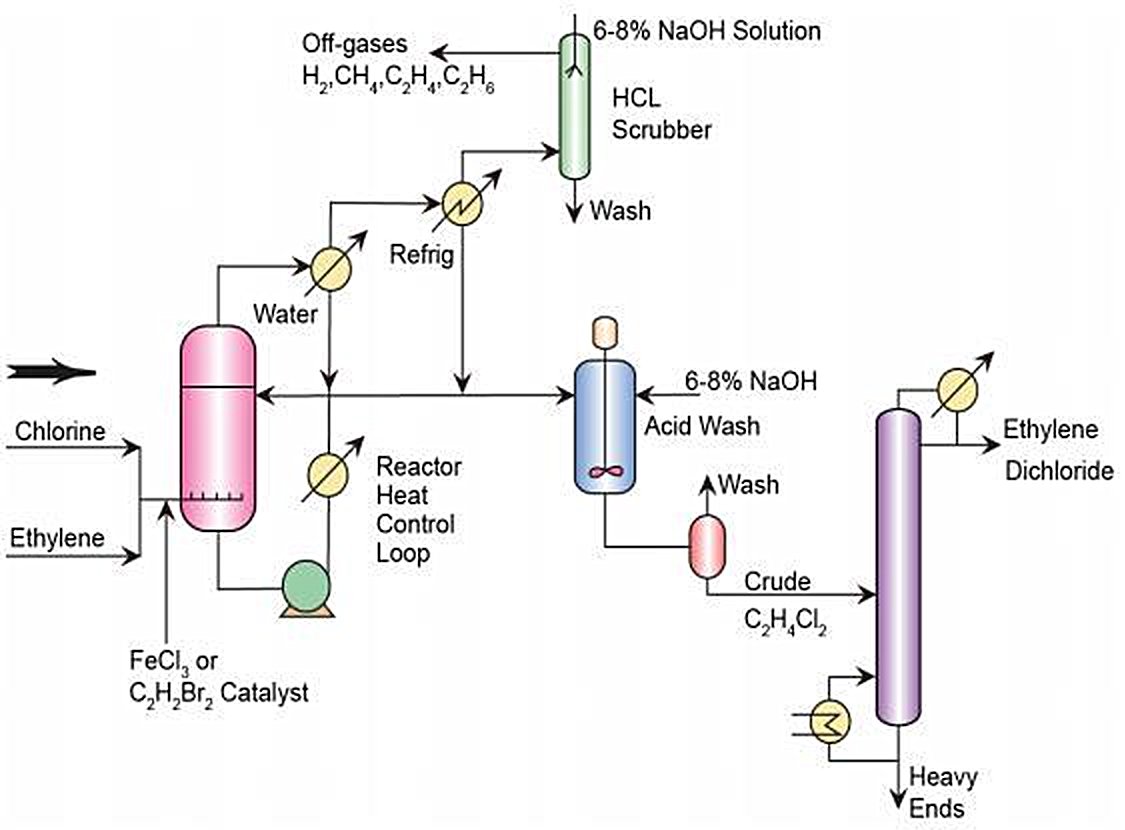
The direct chlorination process of ethylene and chlorine is an exothermic reaction, using the EDC product as the reaction medium.
The operating temperatures are normally 50 - 120°C and the applied pressure ranges from atmospheric to 5 bar.
The reaction takes place in the liquid phase in the presence of a catalyst (usually Fe(III) Chloride).
A slight excess of chlorine or ethylene is preferred to ensure high ethylene conversion.
The reaction product consists of >99% pure EDC. Less than 1% is made up of other chlorinated hydrocarbons, predominantly 1,1,2-trichloroethane and ethyl chloride. To reduce the formation of chlorinated by-products an inhibitor can be added, typically oxygen (especially to reduce the formation of 1,1,2-trichloroethane)[1].
Figure 3 - Direct Chlorination of Ethylene - Simplified Process Flow Diagram[3].
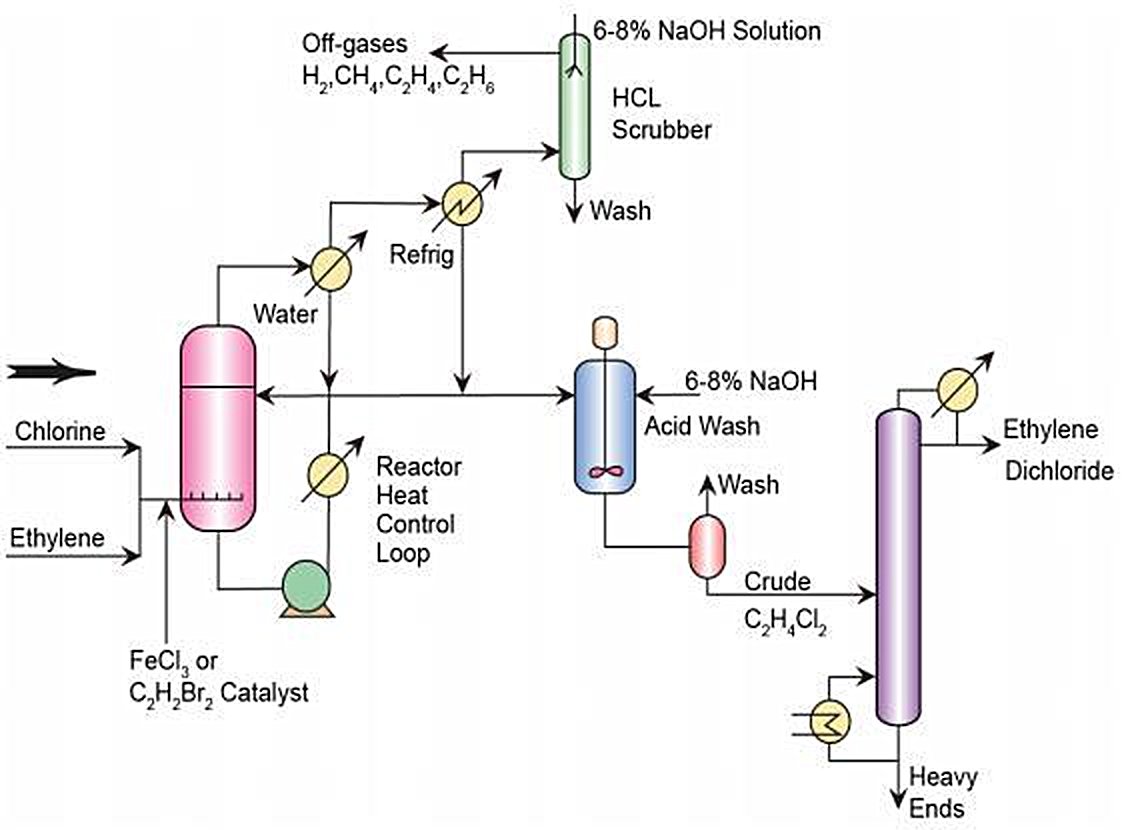
Two variants of the direct chlorination reaction are used: low temperature chlorination and high temperature chlorination.
By 2014, approximately half of the plants in the European Union operate with a low temperature Direct Chlorination design and the rest operate with a high temperature approach[1].
- For low temperature chlorination the reaction is operated below the EDC boiling point at about 20 – 70°C. The heat of the reaction can be dissipated by external cooling, either by means of heat exchangers inside the reactor or by circulation through exterior heat exchangers. The catalysts have to be removed from the liquid EDC when it leaves the reactor. For this purpose, EDC is generally washed and thus requires drying and distillation before cracking to a liquid effluent, which again requires further treatment.
- For high temperature chlorination the reaction is operated above the EDC boiling point. The temperature range is about 85 – 200°C, with a most commonly applied operation temperature at about 100°C. The EDC leaves the reaction section as a vapour and can be directly sent to the EDC cracking unit. In this process, energy may be recovered from the hot vapour[1].
The application of low temperature chlorination produces fewer by-products than high temperature chlorination. However, this process requires considerably higher energy inputs – compared to high temperature chlorination – due to the need for EDC distillation[1].
Oxychlorination of Ethylene
Compared to direct chlorination the oxychlorination process yields less EDC but provides the HCl sink that realises the balanced process[1].
Typically, fluidised bed reactors are used as reaction-technology; fixed beds are also used in some plants[1].
EDC and water are formed by the gas phase reaction of HCl, ethylene and oxygen over a Copper salt catalyst at 220 – 250°C at a pressure of 2 - 6 bar[1]. The EDC molar yield is reported to be 88.41% and 92.47% in different fluidized-bed reactors[4].
Regarding this highly exothermic reaction, temperature control is important to minimise the production of undesirable by-products. The recovered heat of the exothermic reaction is usually used to generate steam[1].
Figure 4 - Oxychlorination of Ethylene - Simplified Process Flow Diagram[5].
.jpeg)
The used HCl has to be of suitable purity. HCl from external sources can be used if the required purity is given. Sources may be the production processes of MDI/TDI, K2SO4, allyl chloride, MCA, DCM or chloroform, where HCl is formed as a by-product (or waste). Additionally, HCl is recycled from the EDC cracking unit and from VCM purification.
The oxygen source can be ambient air, or oxygen, or a mixture of both. The use of air systems results in an increased formation of chlorinated by-products and larger waste streams, whereas oxygen systems require a larger excess of ethylene in the feed and an additional energy input for the production of oxygen[1].
The resulting EDC and the by-products are separated from the inert gases by cooling and condensing at successively decreasing temperatures. After quenching and condensation water and EDC (including other organic chlorinated hydrocarbons) separate naturally into two phases due to the low solubility of EDC and most of the other chlorinated hydrocarbons in water[1].
The oxychlorination stage generates a number of waste streams which do not occur in the case of direct chlorination, comprising impurities removed from the EDC as by-products from the EDC distillation section, process vents requiring treatment, aqueous effluents containing dissolved chlorinated organic compounds, traces of catalyst material in aqueous effluents or spent catalyst, and dioxin related compounds. However, these wastes are not directly emitted into the environment. Instead, further control measures allow for a significant reduction of problematic substances before discharge[1].
EDC purification
EDC products, whether they originate from direct chlorination or oxychlorination, from VCM purification recycling or external sources, have to be purified, since EDC cracking may be susceptible to inhibition and fouling by trace quantities of impurities. Purification may entail washing with water or caustic, azeotropic drying, heavy ends distillation, etc[1].
EDC Cracking
The production of VCM from EDC is achieved by a cracking reaction followed by quenching of the process gas stream.
When subjected to thermal cracking in heated furnaces at temperatures of approximately 500°C, purified EDC splits into VCM and HCl with (single-pass) conversion rates of 50 – 65%.
The pyrolysis gases have to be cooled rapidly to reduce the formation of tars and heavy by-products.
As mentioned above, the purity of the EDC feed has to be very high and greater than 99.5 wt-% to reduce coke formation and fouling of the pyrolysis reactor[1].
Figure 5 - EDC Cracking - Simplified Process Flow Diagram[3].
.png)
VCM purification
After the cracking reaction, HCl and unconverted EDC are separated from VCM by two-stage distillation. Unconverted EDC is transferred back to EDC purification and recycled to the cracking furnacesl[1]. The overall VCM yield after recycling is 95–99%[6].
After an optional hydrogenation stage to remove any remaining traces of acetylene, distilled HCl is recycled as feedstock to oxychlorinationl[1].
Most of the volatile by-products are removed via the HCl flow to oxychlorination. The liquid VCM product is transferred to storage after an optional step to remove the last traces of HCl[1].
References
- Fröhlich, Thomas & Ludmann, Sabrina & Liebich, Axel. (2015). Eco-profiles and Environmental Product Declarations of the European Plastics Manufacturers - Vinyl chloride (VCM) and Polyvinyl chloride (PVC).
- Applied Analytics, Critical Analysis Points in the VCM Balanced Process.
- Rinaldi R. Rachman, Oct 2020, CE4102 - Petrochemical Processes, Lecture 5 - C2 Petrochemicals, Universitas Pertamina, Slideshare upload on 2nd Jun 2022.
- A. Montebelli et al., 8th Sep 2015, Kinetic and Modeling Study of the Ethylene Oxychlorination to 1,2-Dichloroethane in Fluidized-Bed Reactors, Politecnico di Milano.
- Westlake Vinnolit, EDC Oxychlorination Process.
- Joseph Akintola et al., 19th Dec 2024, Optimization and techno-economic evaluation of an integrated process route for the synthesis of vinyl chloride monomer, RSC Sustainability, 2025, 3, 526-539.


.jpeg)
.jpg)

.jpeg)
.png)