Process Description
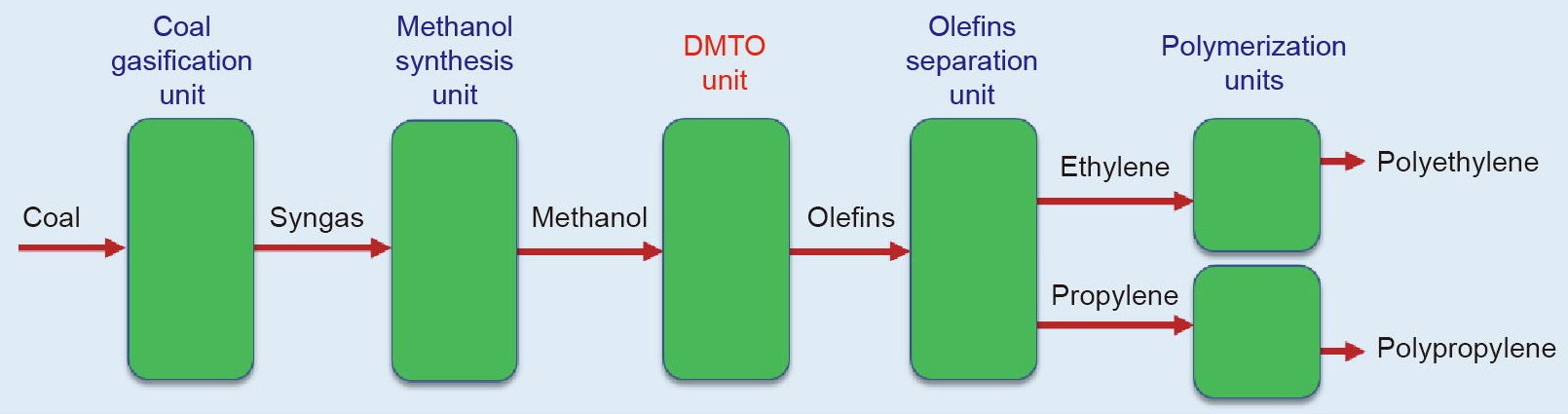
The Methanol-to-Hydrocarbons process was discovered at Mobil Oil in 1977. This process is used to convert methanol to products such as olefins and gasoline. The methanol can first be obtained from coal or natural gas. More generally, a Methanol-to-Olefins MTO) unit is the heart of a modern Coal-to-Olefins (CTO) plant (Fig. 1). Typically, coal is first gasified with steam to produce synthetic gas (CO and H2) in a CTO plant, and then transformed into methanol via a methanol synthesis unit. Through a MTO unit, methanol can be further converted into ethylene and propylene. After passing through a separation unit, the highly purified ethylene and propylene can be used to produce polymers and/or other downstream derivatives.
Figure 1 - Coal-to-Olefins (CTO) plant: (a) provides an overview of the Shenhua Baotou CTO plant, and (b) schematically shows the main units in a CTO plant.
| (a) |
 |
| (b) |
 |
Critical to the successful application of the MTO process are acidic zeolite catalysts. Without these catalysts, the chemical reactions involved in the MTO process would be too slow for the process to be economically feasible. The conversion of methanol to olefins on acidic zeolites takes place through a complex network of chemical reactions.
The distribution of products and thus the “selectivity” depends on the temperature, among other factors. Selectivity is a measure of the amount of one product produced relative to others when the possibility to form multiple products exists. Selectivity depends on temperature through the Arrhenius law for the different rate constants. In general, at lower temperatures methanol reacts to form Dimethyl Ether (DME). At higher temperatures, the desired products (olefins) are produced and the selectivity for DME decreases.
Synthesis Design
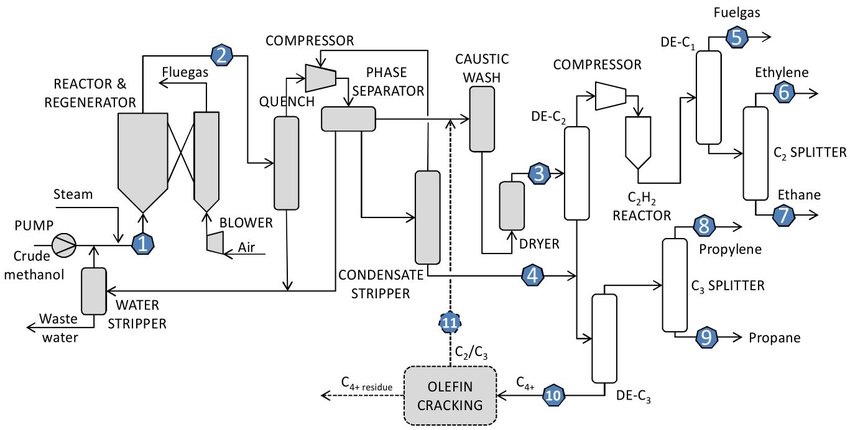
A simplified Block Diagram for a stand-alone MTO Process is shown in Fig. 2. The Fluidised-Bed Reactor/Regenerator system converts feed methanol into a mixture of olefins, which is then fractionated to yield polymer-grade light olefins as major products.
Figure 2 - Simplified Layout of a Stand-Alone MTO Process

- The feed methanol is first compressed to 3 bar, preheated and vaporised in heat exchange with reactor effluent and then mixed with recycled methanol from the downstream process.
- The methanol stream is mixed with steam to increase olefin selectivity and decrease catalyst deactivation in the reactor.
- The combined stream of methanol and water is superheated to 310°C and fed (1) to a fast fluidised MTO reactor operating at 400-450°C and 3 bar.
- In the presence of a proprietary catalyst a nearly complete (99.8 %) conversion of methanol is achieved with ~80% carbon selectivity to ethylene and propylene.
- Coke will gradually build-up on the catalyst surface and to maintain activity, a portion of the catalyst is continuously sent to a combustor (operating at 600°C) where the coke is burned off with air before returning the regenerated catalyst to the MTO reactor.
- The mass ratio between ethylene and propylene in the effluent can be varied from 0.75 to 1.5 by adjusting the operating severity. Higher temperature will lead to more ethylene being produced, although the highest overall yield to light olefins (ethylene plus propylene) is achieved with about equal amounts of both.
Product Recovery and Fractionation
- The reactor effluent (2) is cooled down to 240°C in a feed/effluent heat exchanger and then further to condense the water and unconverted methanol by a scrubber (labelled quench in the flowsheet).
- The recovered methanol is recycled back to the reactor.
- The bottom stream of the stripper contains most of the water contained in the MTO reactor’s effluent and is sent to waste water treatment after exchanging heat with the reactor feed preheater.
- The gaseous effluent is compressed to 25 bar and flashed at 33°C in a phase separator to produce a vapour stream and a condensate stream with two Liquid phases.
- The aqueous phase is separated from the condensate and sent to the stripper while the organic layer is stripped in a separate column and the produced organic concentrate (4) is sent to a downstream depropaniser (labelled De-C3 ).
- Acid gases from the phase separator’s vapour stream are removed by caustic wash.
- The treated acid-free effluent is then cooled to 22°C, dried with a molecular sieve, cooled further to 10°C and sent (3) to a de-ethaniser (De-C2 ) where a majority of ethylene is recovered overhead and most of the propylene from the bottom (condenser temperature -25°C, reboiler 66°C).
- The overhead vapour is compressed to 33 bar and sent through an acetylene converter (C2H2 reactor) where the small amount of acetylene produced in the MTO reactor is hydrogenated to Ethane over a Palladium-based catalyst.
- The treated effluent is then chilled to -20°C and fed to a demethaniser (De-C1 ) that produces methane-rich fuel gas overhead (5) and a mixture of C2 hydrocarbons from the bottom. Very low temperatures (-90°C in the condenser) are needed to carry out this separation.
- The fuel gas is routed through a pressure swing adsorption unit that recovers 86% of the hydrogen contained in the stream.
- After hydrogen Rrcovery the rest of the gas is directed to combustion.
- The C2 stream from the bottom is directed to a C2-splitter column that produces a polymer-grade ethylene stream overhead (6) and an ethane-rich (about 70 mol%) by-product stream from the bottom (7).
- The bottom stream from the de-ethaniser (De-C2) is mixed with the bottoms from the organic layer stripper (condensate stripper) and sent to a depropaniser (De-C3).
- The overhead stream goes to a large C3-splitter producing polymer-grade propylene (8) overhead and a propane-rich (around 60 mol%) by-product (9) from the bottom. The De-C3 bottoms (10) consists of heavy hydrocarbons characterised as a C4+ stream.
Commercial Licenses
Contract Plant numbers and total Olefin capacity of each Licensor are listed in Table 1. China has the most MTO commercial plants (totalling 24 plants) on operation as of March 2021.
Table 1 - Contract Plan Numbers and Total Olefin Capacity / On-Operating Commercial MTO Plant Numbers in China up to March 2021
| Licensor |
SYN
DMTO |
UOP
Hydro-MTO |
Sinopec
S-MTO |
Contract Plant Number
World/China |
26/15 |
8/6 |
5/3 |
Total Olefin Capacity (MMTA)
World/China |
15.25/ 8.36 |
3.52/2.92 |
3.47/2.27 |
Market Share (Olefin-Capacity rel.)
World/China |
68.6/61.7% |
15.8/21.5% |
15.6/16.8% |
References
- Berkeley University of California, Fall 2009, Methanol to Olefins, Based on the MTO paper by Wang et al. (Wang et al. Catalysis Today 113 (2006) 102-114)
- Mao Ye, Peng Tian, Zhongmin Liu, DMTO: A Sustainable Methanol-to-Olefins Technology, Engineering, Volume 7, Issue 1, 2021, Pages 17-21, ISSN 2095-8099.
- Hannula, Ilkka. (2015). Synthetic fuels and light olefins from biomass residues, carbon dioxide and electricity: Performance and cost analysis. 10.13140/RG.2.1.3059.0802.
- Jianghan Shen, Xing Wei, 3 Dec 2020, The Technology of DMTO & DMTO-II, Dalian Institute of Chemical Physics, Division of Low-Carbon Catalysis and Engineering.