Thermochemical Conversion of Biomass
Gasification and Pyrolysis are, at face value, very simple concepts. They were devised over one hundred years ago as Technologies for converting Woody Biomass and Coal into Gaseous and Liquid Chemicals along with producing Carbon-rich Solids. Their names derive from these historical applications. Gasification and Pyrolysis both utilize Thermal Decomposition but avoid Oxidation. They both differ from Direct Combustion that needs Oxygen to be performed. They are designed to inhibit the Combustion Process by limiting Oxygen Ingress while still providing high temperatures, thus stopping Thermal Decomposition prematurely to produce a complex Mixture of unburned Hydrocarbons. Their aim is to convert the Waste into a different Form (Solid, Liquid, or Gas), which is then useable as a Commodity, invariably a "Fuel", in its own right. Extensive research undertaken over the last thirty years has shown that without Catalyst the Pyrolysis or Gasification of Biomass produce Tar as a process-line Contaminant even at Reactor temperatures above 1200 °C. [1], [2]
Figure 1 - Pyrolysis, Gasification and Combustion[2]
.png)
Plastics Gasification Reactor Concepts
Pyrolysis or Gasification techniques are also applied for Thermal Depolymerisation and Cracking of Plastic Waste under reducing conditions. Various types of Reactor Technology have been proposed for commercial or development stage Plastics Gasification, such as a combination of a low-temperature (600-800°C) Fluidized-Bed Gasifier and a high-temperature (1,300-1,500°C), Swirling-Type Slagging Gasifier[3], a Tubular Reactor with a Conveyor Screw made of a material that is associated with means for electrically heating it by the Joule effect[4], a Gasification System utilizing an indirectly heated Medium temperature, low pressure Fluidized Bed Steam Reformer Primary Stage and a higher temperature, low-pressure Fluidized Bed Gasifier Second Stage to generate Syngas[5], a Two-Stage System made of a First Rotating Steel Tubular Reactor where Plastic Chips are gasified at up to 700°C and a Second Tube where Char formed in First Stage is converted into Syngas by reacting it with Steam at 850°C[6] , a Conversion Chamber in which the Plastic is heated to temperatures of more than 850°C.[7]
Syngas from the Gasification Process
Syngas is the main Product of the Gasification Process, created by the Vaporisation of Volatile Compounds from the Raw Material thanks to the heat, which induces a set of complex Reactions. The chemical composition of Syngas and proportion of its Molecules is highly dependent on the Raw Material characteristics and conditions of the Treatment Process. Syngas from low temperature Gasification (700°C - 900°C) is only suitable for energy applications. Syngas from medium temperature Gasification (900°C - 1,650°C) is suitable for Plastic-to-Plastic applications, but only after an additional step to increase the quality of Syngas, which is measured by the H2/CO ratio. Table 1 shows typical Syngas compositions from different Raw Materials when performed under high temperature Pyrolysis conditions, i.e. in the absence of Oxygen.[8]
Table 1 - Examples of Syngas Compositions from High-Temperature Pyrolysis of various Raw Materials[8]
| % Vol |
H2 |
CH4 |
C2-C4 |
CO |
CO2 |
N2 |
LHV# [MJ/Nm3] |
kWh/ton inlet |
| RDF* |
16% |
25% |
24% |
18% |
15% |
2% |
27.3 |
4,690 |
| Biomass |
15% |
26% |
3% |
35% |
17% |
4% |
17.1 |
2,600 |
| Plastics |
25% |
38% |
18% |
9% |
5% |
5% |
28 |
7,800 |
| Tires |
19% |
40% |
28% |
3.5% |
6.5% |
3% |
36 |
3,300 |
*Refuse Derived Fuel; #Lower Heating Value
Feedstock for Plastics Gasification
Plastic Waste Gasification has been performed on Residual House Waste (RHW) collected from the German collection system. This Feedstock needs to be rigorously sorted, cleaned, and dried by removing Organic Food Residues, sorting out of Metals, Cardboard, Paper, etc. A low level of contaminants is as well required so that PET, PVC, PA, PUR, etc. need to be removed. The Gasifier Feedstock is essentially composed of Polyolefin Waste (Polyethylene, Polypropylene and Polystyrene).[9]
Figure 2 - Obtention of Gasifier Feedstock from Residual House Waste[9]

Simulation of Plastic Waste Gasification
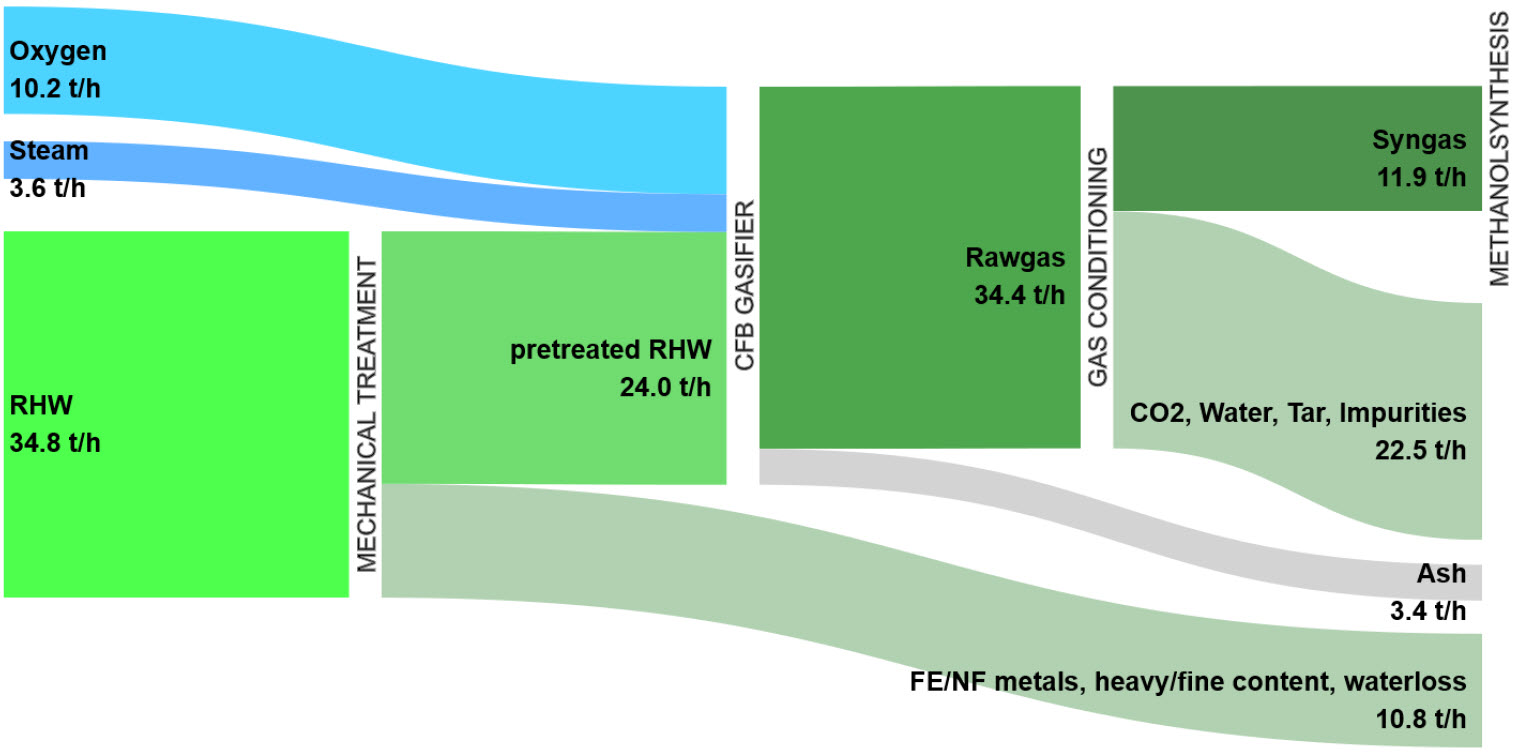
The Gasification of Polyolefin Waste as obtained from RHW is performed in a pilot Continuous Fluidized Bed (CFB) Reactor. The corresponding Process Mass Balance is presented in Fig. 3. Rawgas and Ash are formed in a 91:9 ratio. Syngas must undergo a multi-step Purification Process to remove CO2, Tar and Impurities, and composition rebalancing to yield 31.5% Spec-Syngas (H2:CO = 2:1) suitable for subsequent Methanol Synthesis.[9]
Figure 3 - Mass Balance of Plastic Waste Gasification Simulation in a CFB Gasifier Pilot Plant[9]
References
- B.J. Vreugdenhil and R.W.R. Zwart, Energy Research Center of the Netherlands, Jun 2009, ECN-E--08-087: Tar formation in pyrolysis and gasification
- Andrew N. Rollinson and Jumoke Oladejo, Gaia, June 2020: Chemical Recycling: Status, Sustainability, and Environmental Impact
- Ebara Environmental Plant, Gasification Technologies
- Biogreen®, The Pyrolyzer Spirajoule
- TRI, Gasification
- Plasma Power BV, ECOGY Technologie
- POWERHOUSE ENERGY GROUP, Technology Review, Intellectual Property: GB201910309D0 | GB201910311D0 | GB201910313D0
- Biogreen Energy, Syngas: energy-rich gas for power applications
- Dieter Stapf, 16 Oct 2019, K 2019, Plastics Shape the Future Symposium: Chemical Recycling of Plastic Waste




.png)








