General Process Description
Pyrolysis (or Cracking) can be defined as the chemical and thermal Degradation of Polymeric Materials by heating in the absence of Oxygen in an inert atmosphere e.g. Nitrogen. The Pyrolysis temperature is the most influential Pyrolysis parameter and values between 400 and 800°C are generally employed depending on the Feedstock being processed, whether or not a Catalyst is used, and on the target products.[1]
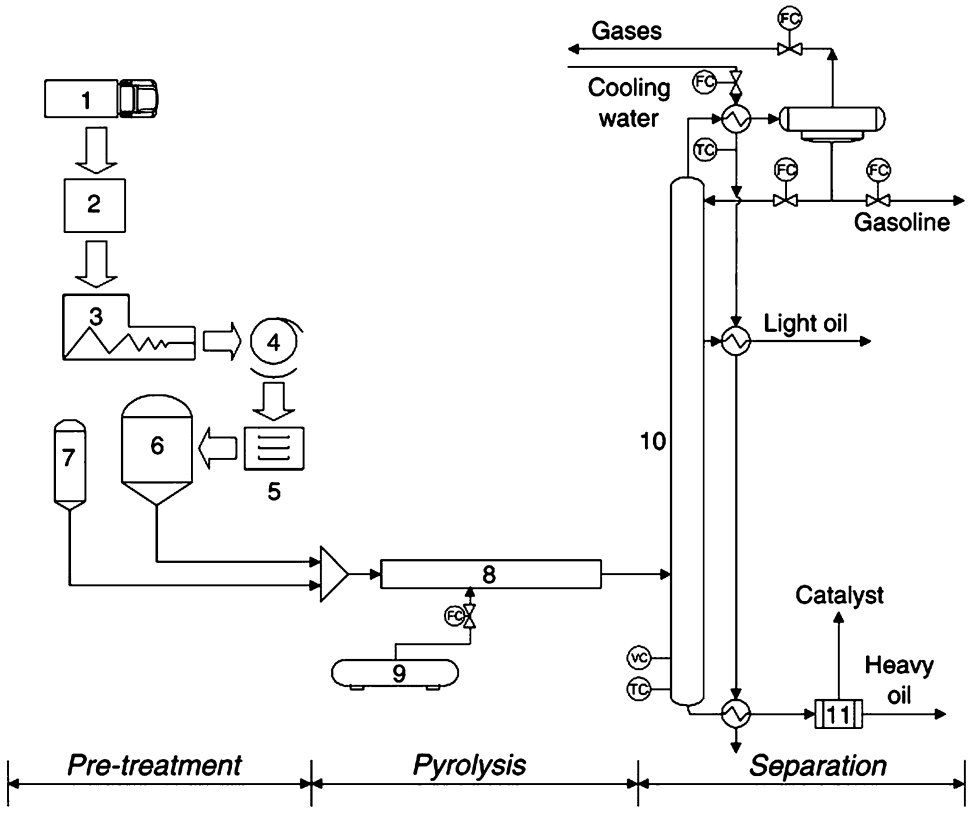
A typical Pyrolysis Process is presented in Fig. 1.
Figure 1 - Schematic of a small-scale Pyrolysis Pilot Plant[1]
Legend: 1-transportation, 2-selective collection, 3-shredding, 4-washing, 5-drying, 6-waste storage, 7-catalyst storage, 8-reactor, 9-heating gas storage, 10--separation unit, 11-catalyst filter
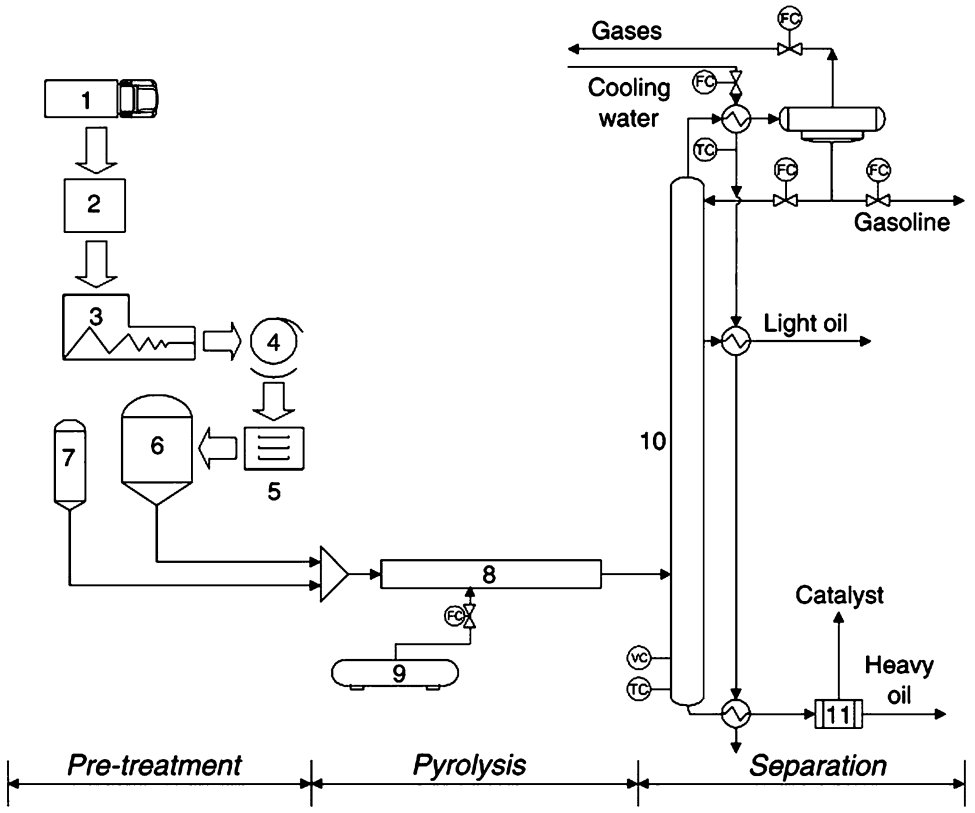
Heating systems for Reactors can be dynamic (e.g. some Batch Reactor configurations) or isothermal (e.g. Fluidised Bed), with isothermal systems being most frequently applied. Various Reactor Types are employed for cracking Plastics are described in Fig. 2.
Figure 2 - Various Types of Reactors used for cracking Plastics[1]
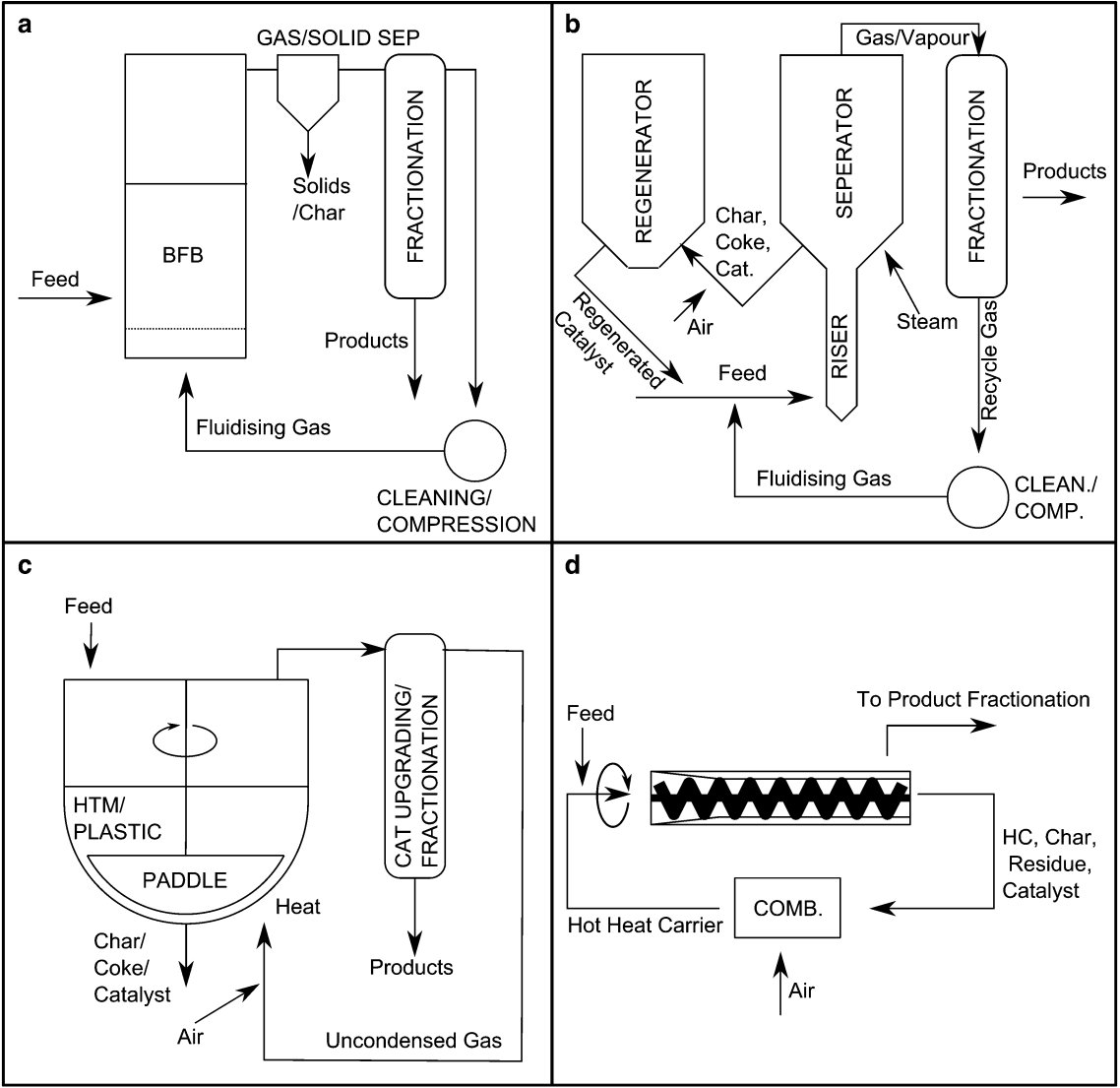
Legend: a bubbling fluidised bed, b fluid catalytic cracker, c stirred tank reactor, d screw/auger reactor (HC= Heat Carrier)
Influence of Operating Conditions
The Process yields carbonised Char and Volatiles that may be separated into Hydrocarbon Oil/Wax and non-condensable Gas[1], which Fraction yields are influenced by the type of Feedstock, operating conditions and the Reactor type as shown in Table 1[2] and Table 2[3].
Table 1 - Summary of example studies investigated as a Chemical Treatment for virgin/waste Polymers in laboratory micro-, bench and pilot scale.[2]
| Scale |
Feedstock |
Process Conditions |
Products |
| Micro |
LLDPE |
20-800 °C, heating rates of 5, 10, 15, 20, 25 °C/min |
Gases |
| Micro |
HDPE |
500-800 °C, heating rate 5 °C/min, nitrogen gas flow, rate 20 ml/min |
Oil, Waxes, Gases, Chars |
| Bench |
HDPE, LDPE, waste LDPE |
400-460 °C, 4-hour reaction time, nitrogen flow rate 5-75 mL/min |
Oil, Polymer Residue (Waxes), Gases, Coke |
| Bench |
Recycled PE |
300-450 °C, residence time 60-180 s |
Gases, Liquid, Solid (non degraded PE), Tar |
| Pilot |
Waste LDPE |
250-400 °C, 60 min reaction time |
Liquid Oil, Gases, Solid Residue |
| Pilot |
Waste HDPE |
465-545 °C |
Gases, Naphtha, Middle Distillates, Heavy Oil |
Table 2 - A summarised representation of the conditions of Plastics Pyrolysis and their potential Products.[3]
| Conditions of Pyrolysis |
Cracking Temperature (°C) |
Heating Rate |
Residence Time |
Derived Products |
| Slow Carbonisation |
450-600 |
Very low |
Over 24 h |
Charcoal |
| Slow Pyrolysis |
450-600 |
10-100 K/min |
10-60 min |
Gas, Oil and Char |
| Fast Pyrolysis |
550-600 |
Up to 1,000 K/s |
0.5-5 s |
Gas, Oil, (Char) |
| Flash Pyrolysis |
450-900 |
Up to 10,000 K/sec |
<1 s |
Gas, Oil, (Char) |
The formation and yields of the various Products formed during the Pyrolysis of Mixed Polyolefin Waste depend largely on operating conditions. As the Pyrolysis temperature of Polyolefins (PO) decreases, increasing Wax and partially converted Feedstock (Residue) Fractions are observed in the yield structure as shown in Fig. 3.[4]
Figure 3 - Products of the Experimental Pyrolysis of Plastic Mixture made of PP 40%, LDPE 35% and HDPE 25%[4]
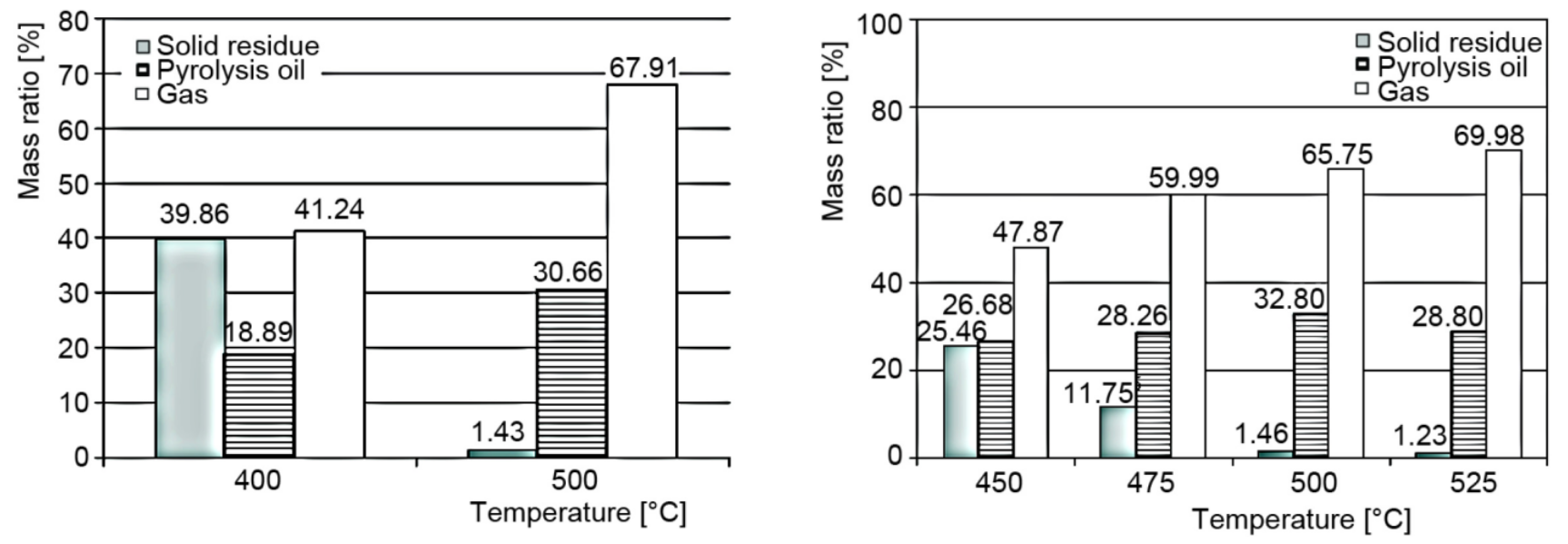
Legend: Left - Reaction time: 60 min, Right - Reaction time: 45 min, (N flow 0.5 Ln per minute, heating rate 12°K per minute).
Waste Plastic Feedstock suitable for Pyrolytic Treatment
From a practical perspective, few Polymers lend themselves to selective Depolymerization: PMMA typically yields about 90% Methyl Methacrylate Monomer[5], and Polystyrene depolymerizes into 65% Styrene and 15% Ethylbenzene under optimized conditions[6]. On the contrary, most other Polymers produce a complex mixture of gazeous, liquid and solid Products when submitted to Thermolytic Treatment conditions, such as Polyester (PET) yielding mostly gaseous Products as a major Fraction and Liquids not exceeding 39% by weight of the total Products, and PVC being mostly gasified - HCl being formed as a main Component or producing a high solid Fraction (Char) depending upon the Reaction conditions as shown in Table 3.[7]
Table 3 - Summary of studies on Plastic Pyrolysis.[7]
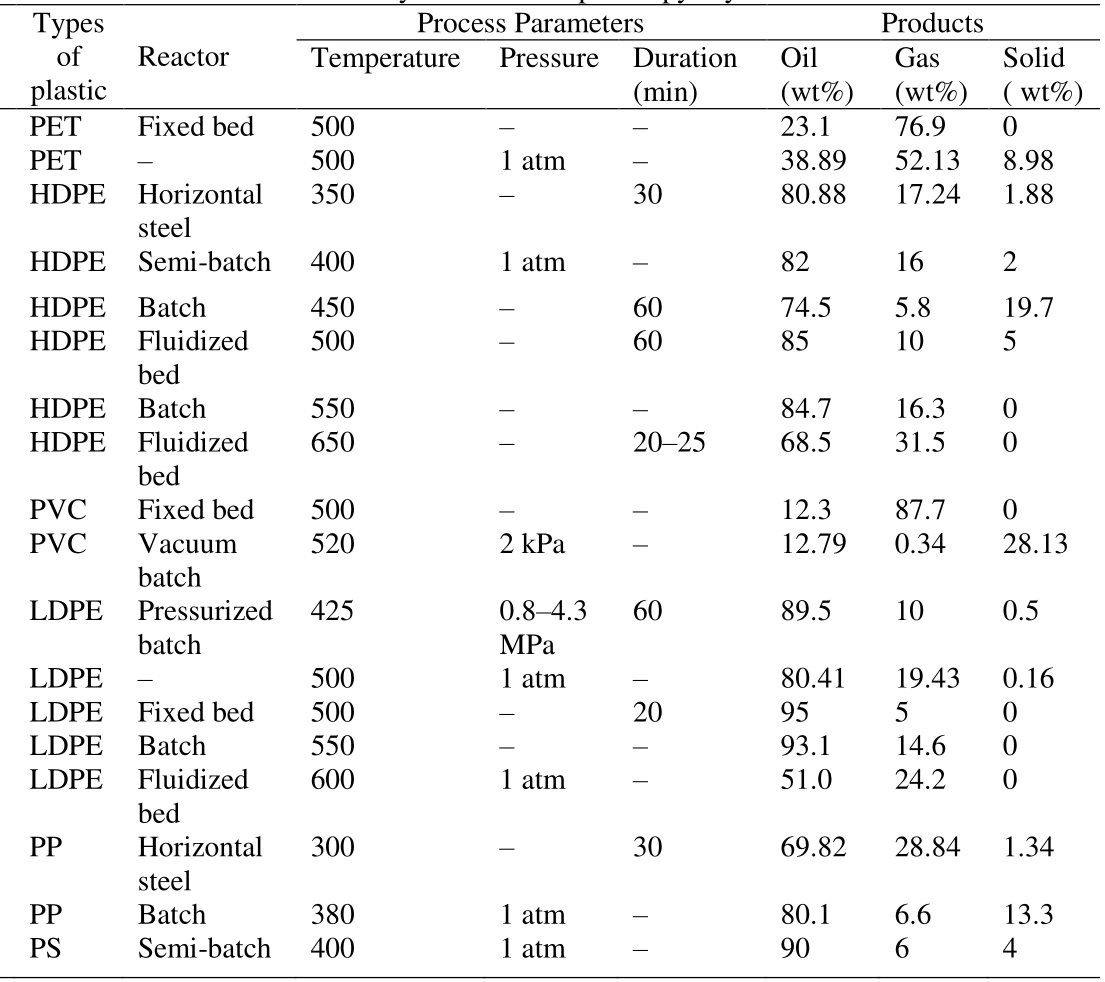
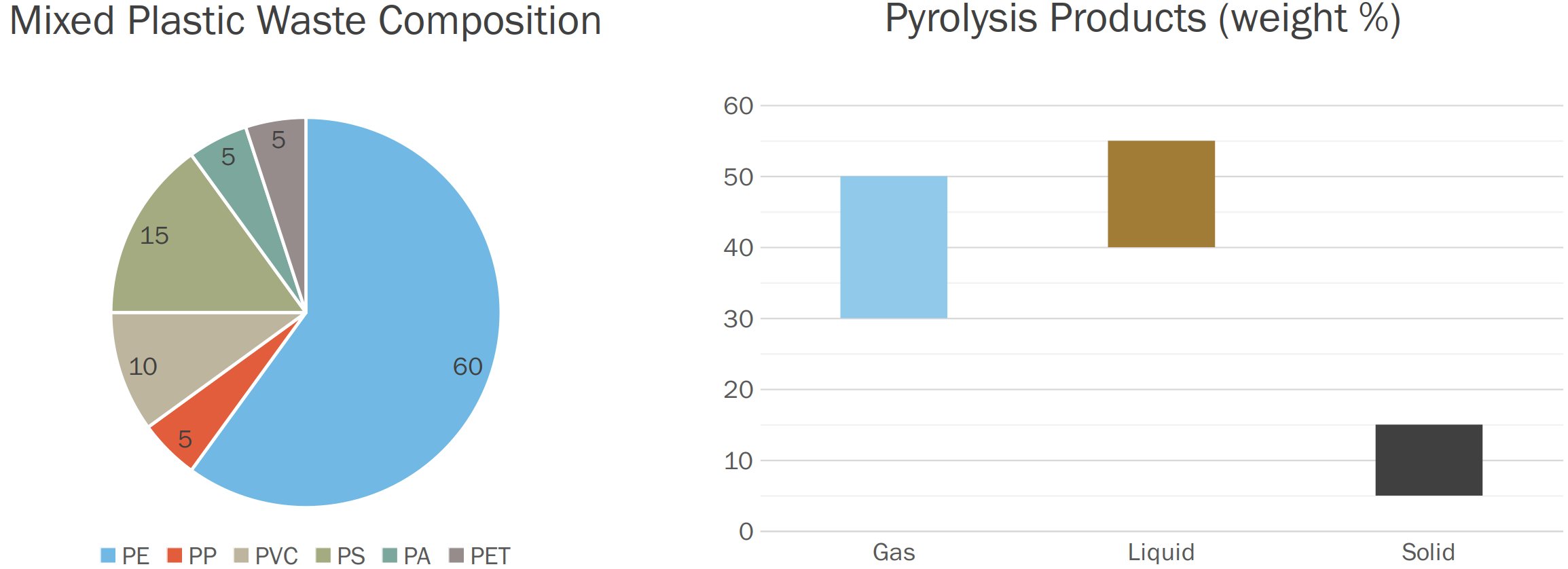
For that reason, Mixed Plastic Waste is not a suitable Feedstock for the Pyrolysis Process as both selectivity and yields of useful Products are too low for commercial purposes as reported in case of the VEBA OIL Technology already 30 years ago[8], the article outlining that a feedstock made of 60% Polyethylene, 15% Polystyrene, 10% PVC and 5% each of Polypropylene, PET and Polyamide yields a Product Mixture composed of 30 to 50% gaseous Products, 40 to 55% Liquids and 5 to 15% Solids by weight (Fig. 4).
Figure 4 - Pyrolysis of Mixed Plastic Waste via the VEBA OIL Process[8]
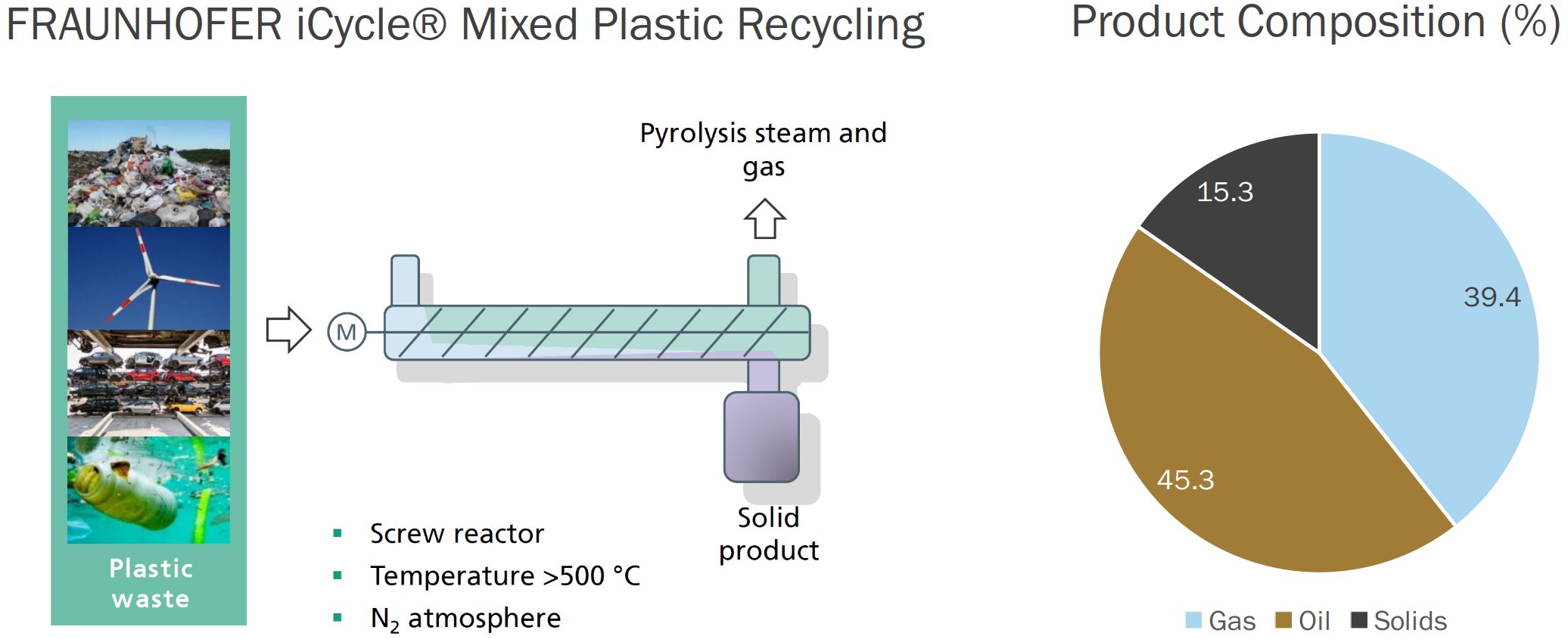
Claims about the Pyrolysis Process being suitable for Mixed Plastic Waste abound, although similarly low selectivity and yields are consistently reported. Such is the case of the FRAUNHOFER iCycle® Technology[9], for which the yields of gaseous, liquid and solid Products from the Pyrolysis of Mixed Plastic Waste are reported to be respectively 39.4%, 45.3% and 15.3% by weight (Fig. 5).
Figure 5 - Pyrolysis of Mixed Plastic Waste via the FRAUNHOFER iCycle® Process[9]
It is publicly acknowledged that commercial-stage Pyrolysis plants operate with Mixed Polyolefin Waste as a Feedstock, obtained via either dedicated collection schemes or selective sorting and cleaning of Mixed Plastic Waste. From the Conversion of this Mixed Polyolefin Waste, the following yields have been reported (Tab. 4):
Table 4 - Published Yields of Mixed Polyolefin Waste Pyrolysis
| Plastic Energy Ltd. TAC[10] |
Quantafuel Plastics-to-Liquids[11] |
Nexus Circular Technology[12] |
- The approx. 72-75% TACOIL produced is sold to the petrochemical industry
- The approx. 18% syngas produced is used to power the plant and reduces the need for outside energy
- The approx. 8-10% Char produced is sold to the construction industry
|
Pyrolysis yields are about
- 10% permanent gases and four different products
- 10% of an ash fraction (70 wt-% carbon)
- 16 wt-% light fraction (C6-C12), 56% diesel fraction (C11-C21), and 8% heavy fraction (C20-C28) Summing up to 80%*
|
Plastic pyrolysis forms
- gas
(7-10%)
- liquid (72-79%)
- wax
(3-10%)
- char
(4-8%)
|
*In a subsequent communication[13], Quantafuel reports about an overall Oil yield of 68% instead of the 80% claimed in the company's Q4 2019 report.
Pyrolysis Products and Post-Processing Requirements
Thermal Cracking of Polyolefins is usually carried out either at high temperatures (>700°C), to produce an Olefin mixture of C1–C4 Gases and Aromatic Compounds (Benzene, Toluene and Xylene) or at low temperatures (400–500°C) where the yield structure comprises a high-calorific value Gas, condensable Hydrocarbon Oils and Waxes.[1] It is noteworthy that commercial Processes are mostly geared towards Liquid Production under high-temperature conditions (Fast Pyrolysis), rather than towards the Production of Waxes under low-temperature conditions (Slow Pyrolysis) probably due to economic imperatives such as to optimize the Production of liquid Hydrocarbons with a typical yield of 70-75% by weight as mentioned in the previous section. This Liquid Fraction is called Pyrolysis Fuel Oil, also commonly denominated Pyrolysis Oil or PyOil, a form of Gasoil (Diesel) as it covers a broad range of Hydrocarbon Molecules from C6 to C28 as presented in Tab. 4. This PyOil is largely made of olefinic Compounds[14] and requires both Hydrotreatment to saturate the Olefin Groups and Fractionation of the Hydrotreated PyOil to isolate the lighter Components (Naphtha, Diesel) before they can be returned to the Steam Cracker for conversion into Monomers such as Ethylene, Propylene and Butadiene.
References
- Butler, E., Devlin, G. & McDonnell, K. Waste Polyolefins to Liquid Fuels via Pyrolysis: Review of Commercial State-of-the-Art and Recent Laboratory Research. Waste Biomass Valor 2, 227–255 (2011).
- Charlotte Abdy, Yuqing Zhang, Jiawei Wang, Yang Yang, Ignacio Artamendi, Bob Allen, Pyrolysis of polyolefin plastic waste and potential applications in asphalt road construction: A technical review, Resources, Conservation and Recycling, Volume 180, 2022,106213, ISSN 0921-3449.
- Yansaneh, O.Y.; Zein, S.H. Recent Advances on Waste Plastic Thermal Pyrolysis: A Critical Overview. Processes 2022, 10, 332.
- Papuga, Saša & Gvero, Petar & Vukic, Ljiljana. (2015). Temperature and time influence on the waste plastics pyrolysis in the fixed bed reactor. Thermal Science. 20. 154-154. 10.2298/TSCI141113154P.
- W. Kaminsky * and J. Franck: Monomer recovery by pyrolysis of poly( methyl methacrylate) (PMMA), Journal of Analytical and Applied Pyrolysis, 19 (1991) 311-318.
- Ali Karaduman: Pyrolysis of Polystyrene Plastic Wastes with Some Organic Compounds for Enhancing Styrene Yield, Energy Sources, 24:7, 667-674, (2002).
- S D A Sharuddin et al, Pyrolysis of plastic waste for liquid fuel production as prospective energy resource, 2018 IOP Conf. Ser.: Mater. Sci. Eng. 334 012001.
- 6. H.P. Wenning: The VEBA OEL Technologie pyrolysis process, Journal of Analytical and Applied Pyrolysis, 25 (1993) 301-310.
- ONLINE Plastic Waste 2 Plastic Conference, Fraunhofer UMSICHT, Dr. Alexander Hofmann, 9 Nov 2020: Chemical Recycling of Plastic Waste –Pyrolysis and downstream processing of pyrolysis oils
- Plastic Waste 2 Plastic Conference, Plastic Energy, Carlos Monreal, Founder & CEO, 9 Nov 2020: Chemical Recycling for the Circular Economy: Transforming Plastic Waste into Virgin-Quality Plastics
- QUANTAFUEL, 4Q 2019 report (no longer available online)
- Georgia Recycling Coalition Inc., 2015, Nexus Fuel, LLC: Plastic Waste to Fuel.
- SUSTAINABLE Plastics, Karen Laird, 28th Mar 2022, Proof of Concept announcement from Norwegian chemical recycler Quantafuel.
- Shafferina Dayana Anuar Sharuddin, Faisal Abnisa, Wan Mohd Ashri Wan Daud, Mohamed Kheireddine Aroua, A review on pyrolysis of plastic wastes, Energy Conversion and Management, Volume 115, 2016, Pages 308-326, ISSN 0196-8904.