1. Process History
The cumene oxidation process, also known as the Hock Process, is the principal industrial method for producing phenol and acetone. The process was first discovered and developed in the early 1940s by Russian scientists Rudolf Yur’evich Udris and Petr Sergeyev. In 1942, Udris was the first to isolate cumene hydroperoxide (CHP) from cumene oxidation products and to demonstrate its acid-catalyzed conversion to phenol and acetone. This work was followed by the development of the first industrial process for cumene oxidation and CHP cleavage by Udris, Mark Nemtsov, and Boris Kruzhalov, with patents filed in Russia in 1947[1].
Independently, in 1944, German chemist Heinrich Hock discovered the same acid-catalyzed cleavage reaction of cumene hydroperoxide to phenol and acetone. The process and the key rearrangement reaction are named after Hock, and the term "Hock Process" is widely used in the literature[1].
2. Process Summary
The Hock Process involves two main steps:
-
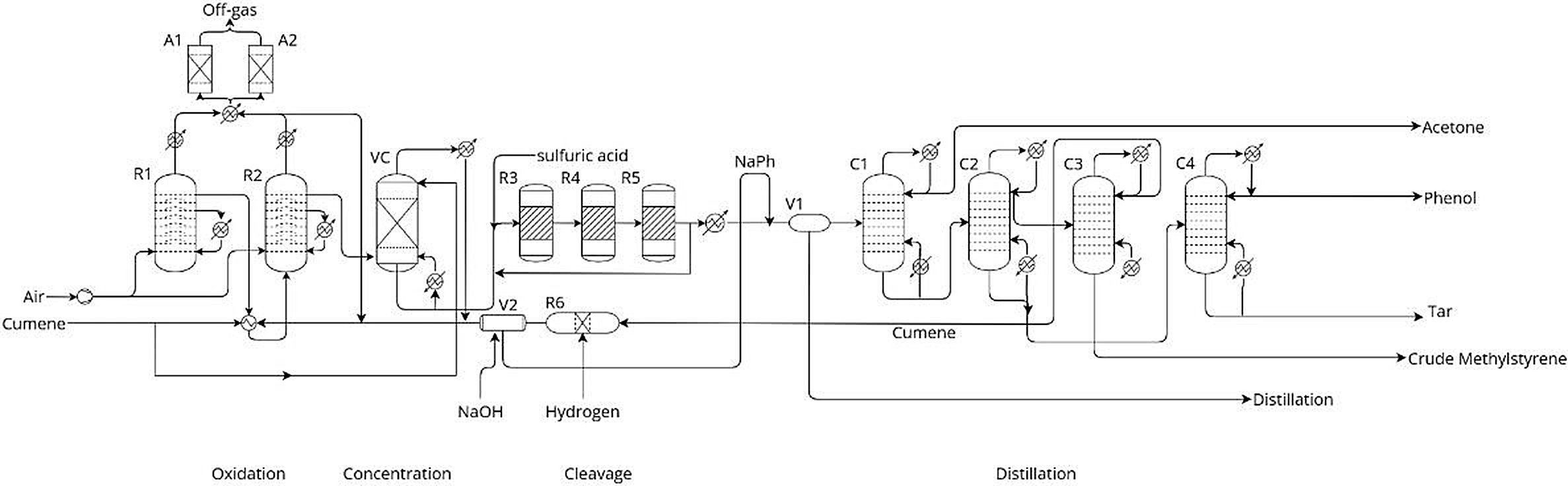
Oxidation of cumene (isopropylbenzene) to cumene hydroperoxide (CHP)
C6H5CH(CH3)2 + O2 → C6H5C(CH3)2OOH
(Cumene + Oxygen → Cumene Hydroperoxide)
-
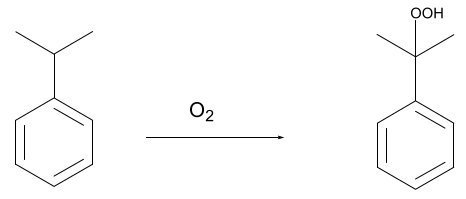
Acid-catalyzed cleavage (Hock rearrangement) of CHP to yield phenol and acetone
C6H5C(CH3)2OOH → C6H5OH + CH3COCH3
(Cumene Hydroperoxide → Phenol + Acetone)
This process is favored for its high selectivity, relatively mild operating conditions, and the simultaneous production of two valuable chemicals from a single feedstock[1][3][4].
4. Step-by-Step Process Description
4.1. Process Flow Overview
- Cumene Oxidation:
- Cumene is oxidized in the liquid phase with air (oxygen) in a series of reactors.
- The reaction is a free-radical chain process, typically conducted at 80–100°C and moderate pressure.
- Cumene hydroperoxide (CHP) is formed as the main product (10–25% per reactor), with minor by-products such as dimethylphenylcarbinol (DMPC) and acetophenone (ACP)[1][3].
- CHP Concentration:
- The reaction mixture is concentrated, often by vacuum distillation, to increase CHP content to 70–80% before cleavage.
- Cleavage (Hock Rearrangement):
- The concentrated CHP is mixed with an acid catalyst (commonly sulfuric acid) in a cleavage reactor.
- The exothermic reaction cleaves CHP into phenol and acetone.
- The process must be carefully controlled due to the hazardous and exothermic nature of CHP decomposition[5][6].
- Neutralization:
- The acidic effluent is neutralized, typically with caustic soda, to prevent corrosion and side reactions.
- Product Separation and Purification:
- The mixture is separated by distillation into phenol, acetone, unreacted cumene (recycled), and by-products.
- Phenol and acetone are further purified to meet commercial specifications.
- By-products such as alpha-methylstyrene (AMS) may be recovered and sold or recycled[7][8].
- Effluent Treatment:
- Wastewater and other effluents are treated to meet environmental regulations.
4.2 Process Flow Diagram
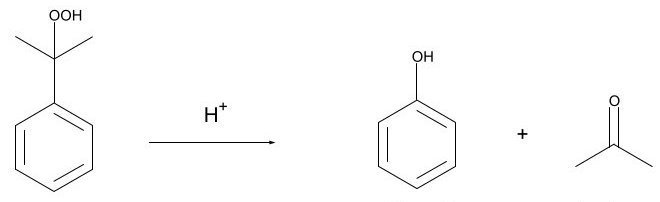
The process flow diagram of the production of phenol-acetone from cumene is shown in the following figure[5]:
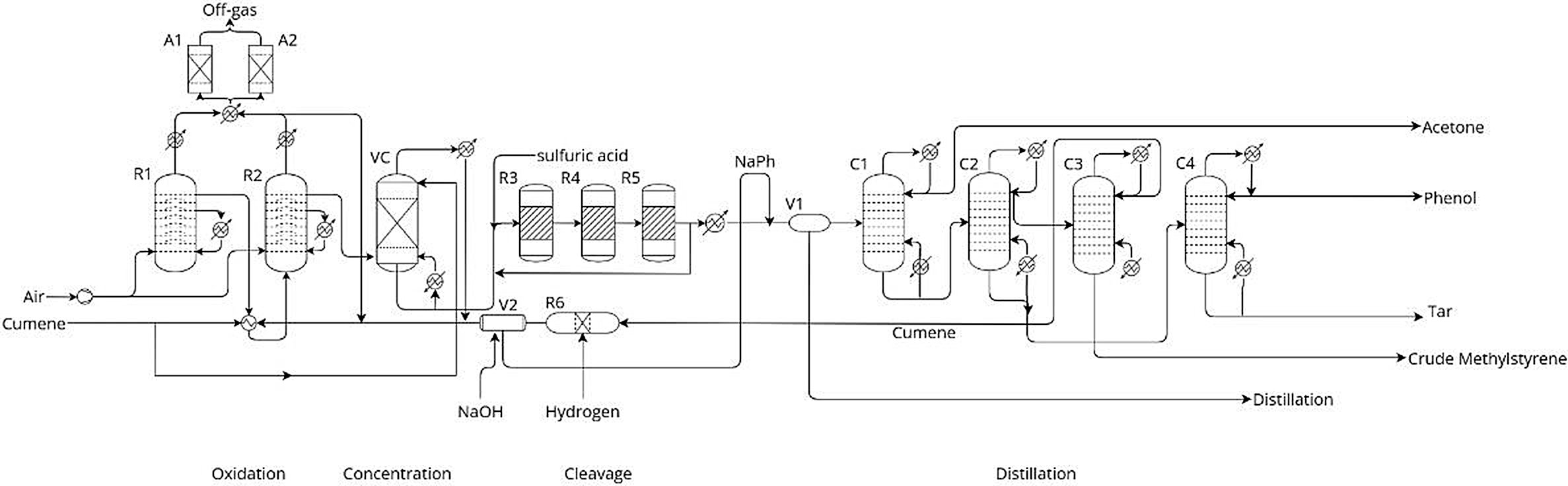
Legend: R1, R2: cumene oxidation reactors; A1, A2: activated-carbon adsorbers; VC: vacuum distillation column; R3-R5: cleavage reactors; V1: neutralization drum; C1: acetone column; C2: cumene column; C3: a-methylstyrene column; C4: phenol column; R6: hydrogenation reactor; V2: cumene scrubber.
5. Process Efficiency
- Selectivity: Modern plants achieve phenol selectivity of 95–97% (mol) based on cumene[1][2].
- Yield: Overall yields for phenol and acetone are high, with minimal by-product formation.
- Energy Consumption: The process is energy efficient, especially with heat integration and modern reactor designs.
- By-products: Main by-products include alpha-methylstyrene (AMS), acetophenone, and phenol tar. AMS can be recovered and sold or hydrogenated back to cumene[2][9].
- Safety: CHP is hazardous and requires careful handling and process control due to its explosive potential[5].
6. Economic Performance
- Capital and Operating Costs: The Hock process is capital efficient due to its relatively simple flowsheet and the co-production of two valuable products.
- Product Value: The economic viability depends on the market demand and price ratio of phenol to acetone. The process is most profitable when both products are in demand[7][10].
- By-product Credits: Sale or recycling of AMS and other by-products can improve process economics.
- Process Improvements: Recent advances focus on reducing waste, improving selectivity, and minimizing energy use[9][10].
7. Technology Licensors
Several companies license cumene and phenol/acetone technology globally, including in China:
References
- V. M. Zakoshansky, The Cumene Process for Phenol–Acetone ProductionPetroleum Chemistry, 2007, Vol. 47, No. 4, pp. 273–284, ISSN 0965-5441, DOI: 10.1134/S096554410704007X.
- Wikipedia, Cumene process.
- Cátia Folgado Saturnino Gordicho da Costa, 2nd Dec 2009, Master degree dissertation: Cumene oxidation to cumene hydroperoxide, Universidade Técnica de Lisboa.
- Hock Process - an overview | ScienceDirect Topics.
- Jan Drönner et al., Solid Acid Catalysts for the Hock Cleavage of Hydroperoxides. Catalysts 2022, 12, 91, DOI: 10.339/catal120110091.
- Chris Heil, Thermo Fischer Scientific, 2008, Application Note: 5171: FT-NIR Analysis of the Hock Process for the Production of Phenol and Acetone.
- Tiffany Robinson et al., Weebly, Poster CHF 100: Phenol Production Process Design, Louisiana State University, Document date: 27th Apr 2015.
- Nurul Hazwani et al., 14th Mar 2011, Production-of-phenol.
- Vladimir Mikhailovitch Zakoshansky, Irina Ivanovna Vassilieva, US Patent No. US 6,252,124 B1, Wasteless economic method of production of phenol and acetone, Priority date: 29th Apr 1999, Assignee: ILLA International LLC.
- Pedro G. Junqueira et al., Economic and environmental analysis of the cumene production process using computational simulation, Chemical Engineering and Processing - Process Intensification, Vol. 130, pp. 309-325, 2018, ISSN 0255-2701, DOI: 10.1016/j.cep.2018.06.010.