The Friedel-Crafts alkylation
The Friedel-Crafts alkylation of aromatic compounds involves an acid-catalyzed electrophilic substitution, wherein an alkyl group replaces an aromatic hydrogen. A diverse range of alkylating agents, such as olefins, alkyl halides, and alcohols, is commonly employed for this purpose.
This reaction can be applied to various aromatic substrates, including heteroaromatic compounds and even compounds like ferrocene. These reactions are typically rapid and exothermic, and they are often conducted under mild conditions in the liquid phase.
However, in some cases, vapor-phase processes with more stringent conditions are utilized for certain substrates. When olefins, alkyl halides, and alcohols are used as alkylating agents.
Catalysts used in the Friedel-Crafts alkylation process are acidic, and their effectiveness depends on various factors, including reaction conditions, alkylating agents, and aromatic substrates. Both Lewis acids and Brønsted acids can act as active catalysts, and frequently used catalysts are classified based on their chemical structures[1].
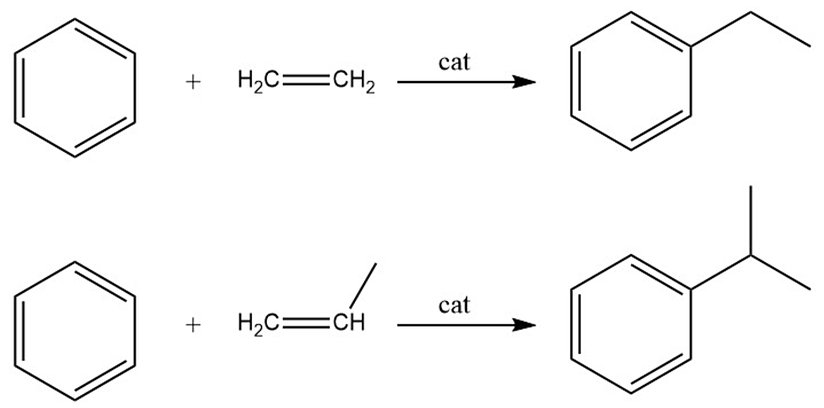
Alkylation of Benzene
The alkylation of benzene finds three major commercial applications:
- Alkylation with ethylene to produce ethylbenzene, which serves as an intermediate for styrene production.
- Alkylation with propene to produce cumene, which is an intermediate for phenol production, with acetone as a byproduct.
- Alkylation with long-chain olefins containing 10 – 18 carbon atoms to produce alkylbenzenes, which are then sulfonated to produce surface-active compounds named linear alkylbenzenesulfonates (LAB)[1].
Alkylation of Benzene with ethylene
The industrial production of ethylbenzene through the alkylation of benzene with ethylene represents one of the most significant petrochemical processes globally, serving as the primary feedstock for styrene production. This process has evolved dramatically since its initial commercial development in the 1930s, transitioning from corrosive acid catalysts to modern zeolite-based systems that offer superior efficiency, environmental performance, and economic advantages.
Process History and Development
The ethylbenzene synthesis process was first developed commercially in the 1930s in Germany and the United States[2]. The earliest industrial processes utilized aluminum chloride (AlCl3) as a homogeneous catalyst in liquid-phase alkylation, operating at low temperatures (85-95°C) and atmospheric pressure[3]. However, these processes faced significant challenges including catalyst corrosivity, environmental concerns, and complex handling requirements.
The development of solid phosphoric acid (SPA) catalysts in the 1940s marked the first major advancement, offering a heterogeneous alternative with reduced corrosivity[3]. These catalysts, typically supported on diatomaceous earth, operated at higher temperatures and pressures.
The revolutionary breakthrough came in the 1980s with the introduction of zeolite-based catalysts, particularly the Mobil-Badger vapor-phase process using ZSM-5 catalyst commercialized in 1981[5]. This was followed by the development of liquid-phase processes using zeolite catalysts, including the EBMax process utilizing MCM-22 family catalysts commercialized in 1995. Today, zeolite-based processes account for the majority of global ethylbenzene production, with plants using Badger Ethylbenzene technologies producing over half of the world's ethylbenzene capacity.
Chemical Reactions and Process Summary
The ethylbenzene synthesis process involves the Friedel-Crafts alkylation of benzene with ethylene, proceeding through an electrophilic aromatic substitution mechanism. The primary reaction produces ethylbenzene as the desired product:
Main reaction:
C6H6 + C2H4 → C6H5–C2H4 (Cumene)
Side reaction:
C6H5–C2H4 + C3H6 → C6H4(C2H5)2 (Diisopropylbenzene, DIPB)
Transalkylation Reaction:
C6H4(C2H5)2 + C6H6 → 2 C6H5CH3CH7 (Converting DIPB back to cumene)
The reaction mechanism proceeds through carbonium ion intermediates, where ethylene forms a carbonium ion that attacks the benzene ring. The process is highly exothermic with typical heat of reaction around -20 kcal/mol[3]. The selectivity to monoethylated product (ethylbenzene) versus polyethylated products (diethylbenzene and higher) depends strongly on the benzene-to-ethylene ratio, with higher ratios favoring ethylbenzene formation[5].
Alkylation of Benzene with Propene
The industrial production of cumene (isopropylbenzene) through the alkylation of benzene with propylene represents one of the most important petrochemical processes worldwide, serving as the primary feedstock for phenol and acetone production via the cumene process[6]. This process has evolved significantly since its initial development during World War II to meet aviation fuel demands, transitioning from traditional acid catalysts to modern zeolite-based systems that offer superior efficiency, environmental performance, and economic advantages[7].
Process History and Development
The cumene synthesis process was originally developed between 1939 and 1945 to produce high-octane aviation gasoline during World War II[7]. The earliest commercial processes utilized various acid catalysts including aluminum chloride, phosphoric acid, and boron trifluoride for the alkylation reaction[7]. However, the most significant advancement came in the late 1980s with the introduction of zeolite-based catalysts, particularly beta zeolite technology developed by UOP and MCM22 catalysts from ExxonMobil[7][8].
The breakthrough occurred when workers at Chevron invented a liquid phase alkylation process using beta zeolite catalyst in 1988, patented in 1990[7]. This innovation led to the development of modern processes such as UOP's QMax technology using QZ2000 and QZ2001 catalysts, and ExxonMobil's Badger Cumene Technology using MCM22 catalysts[7][8]. Today, over 80% of all cumene is produced using zeolite-based processes, which have largely replaced older technologies due to their superior performance characteristics[7].
Chemical Reactions and Process Summary
The cumene synthesis process involves the Friedel-Crafts alkylation of benzene with propylene, proceeding through a modified reaction mechanism. The primary reaction produces cumene (isopropylbenzene) as the desired product[7].
Main reaction:
C6H6 + C3H6 → C6H5–C3H7 (Cumene)
Side reaction:
C6H5–C3H7 + C3H6 → C12H18 (Diisopropylbenzene, DIPB)
Transalkylation Reaction:
C12H18 + C6H6 → 2 C6H5–C3H7 (Converting DIPB back to cumene)
The reaction mechanism proceeds through carbonium ion intermediates, where propylene forms a carbonium ion that attacks the benzene ring in an electrophilic substitution reaction[6]. The addition follows Markovnikov's rule, with the propylene attaching at the middle carbon of the olefin double bond[6]. The process is highly exothermic with a heat of reaction of 23.4 kcal/mol at 250°C[9].
References
- Chemcess, 5th Sep 2024, Alkylation of Aromatic Compounds.
- Hashim Khan, Ethylbenzene presentation, Slideshare.
- Prasannan Kumar Sahoo, 2011, Thesis: Production of Ethylbenzene by Liquid-Phase Benzene Alkylation, National Institute of Technology Rourkela.
- V. N. Ipatieff and Louis Schmerling, Ethylation of Benzene in Presence of Solid Phosphoric Acid, Industrial & Engineering Chemistry 1946 38 (4), 400-402, DOI: 10.1021/ie50436a017.
- Thomas F. Degnan et al., Alkylation of aromatics with ethylene and propylene: recent developments in commercial processes, Applied Catalysis A: General, Volume 221, Issues 1–2, 2001, Pages 283-294, ISSN 0926-860X, DOI: 10.1016/S0926-860X(01)00807-9.
- Robert J. Schmidt, UOP LLC, Cumene Production, 2006, Encyclopedia of Chemical Processing DOI: 10.1081/E-ECHP-120026490.
- Engineers Guide Blogspot, Cumene production flow sheet and Process description.
- Wikipedia, Cumene process.
- Shiyong Xing et al., Elucidation of the mechanism and structure–reactivity relationship in zeolite catalyzed alkylation of benzene with propylene, Catal. Sci. Technol., 2021, Vol. 11, Issue 8, pp. 2792-2804, The Royal Society of Chemistry, DOI: 10.1039/D0CY02374D.