Technology Type
- Type
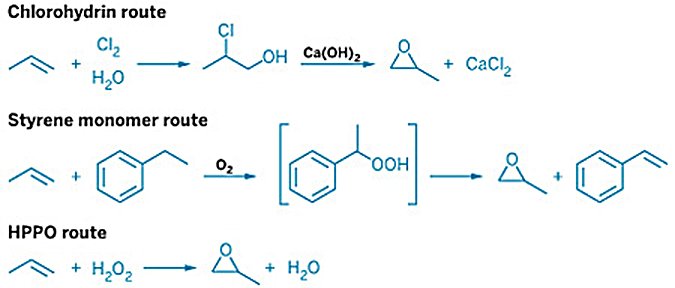
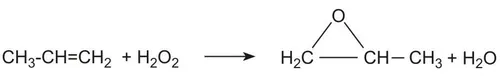
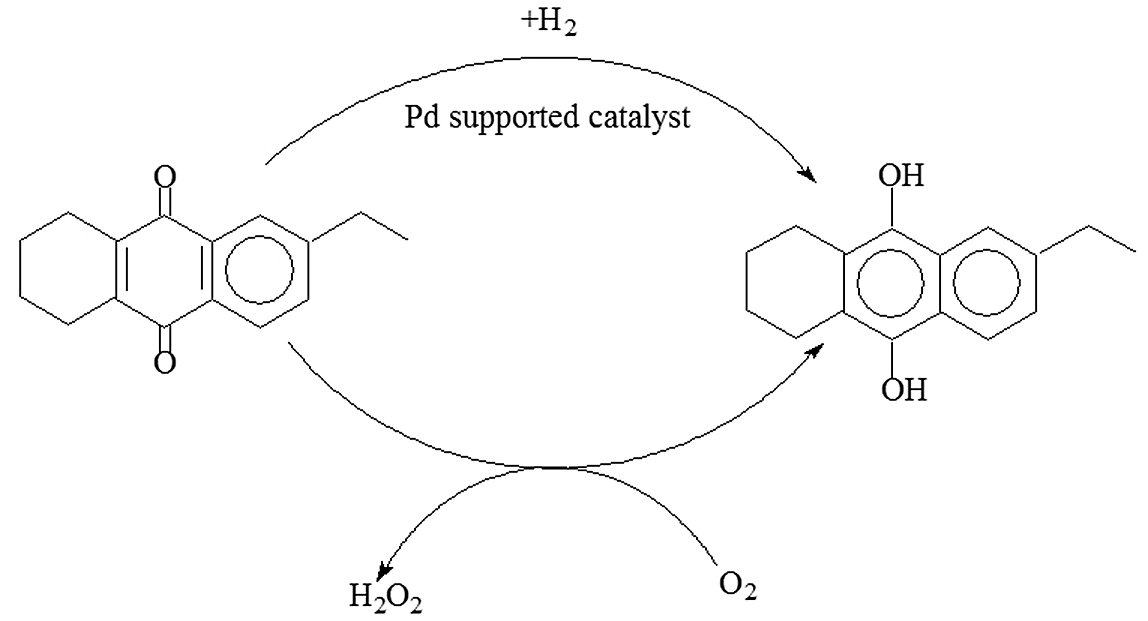
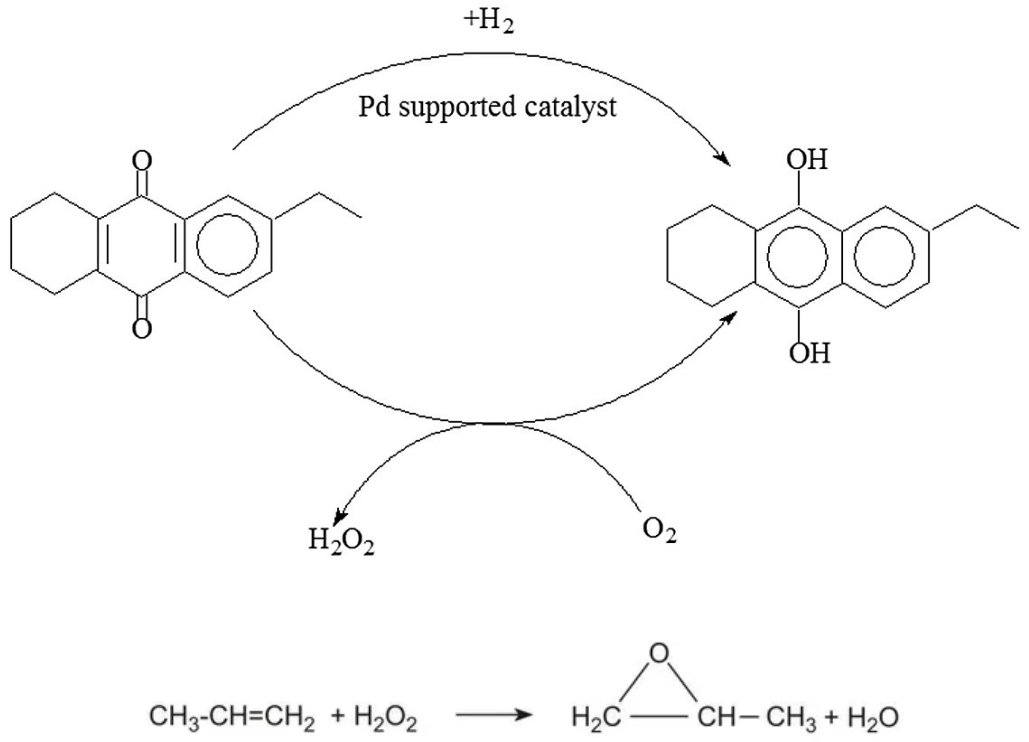
- Direct Oxidation Routes for Propylene Oxide Production
- Process
- Peroxidation
-

- #TT54
Description
Your insights will be shown here
Image
| Technology | Owner | Entity |
|---|---|---|

|
BASF Dow |
Content provided by
| Transaction | Name | Date |
|---|---|---|
| Modified by |
|
7/14/2025 10:31 AM |
| Added | 3/6/2022 12:11 PM |