Acid Gas
Acid Gas Removal (AGR) or Gas Sweetening is the process of removing hydrogen sulfides, carbon dioxide, and mercaptans from natural gas to make it suitable for transport and sale. Sour gas must be sweetened because hydrogen sulphide (H2S) and carbon dioxide (CO2) have a corrosive effect on gas pipelines and are also toxic to humans. In the US, sub-quality gas (gas requiring upgrading or blending) was defined as containing greater than 2% CO2, greater than 4% total inerts (which includes both carbon dioxide and nitrogen), and greater than 4 parts per million of H2S, and 41% of proven gas reserves and 34% of gas production fall into this category.
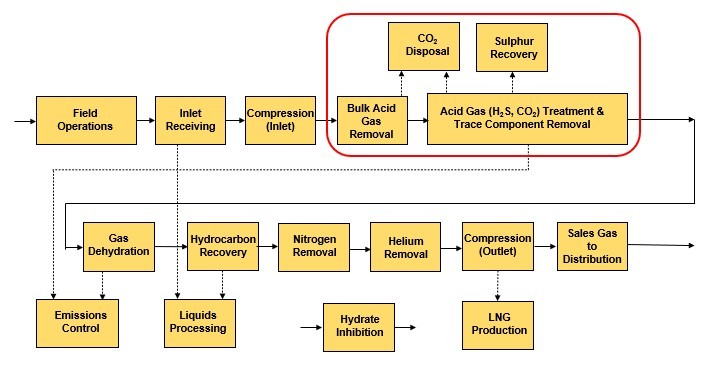
Although many natural gases are free from objectionable amounts of H2S and CO2, substantial quantities of these impurities are found in both gas reserves and production throughout the world. For that reason, most gas processing facilities have a gas treating step to remove these acid gases (Fig. 1).
Figure 1 - Acid Gas Removal in a Gas Processing Facility
However, it is not just the outlet product specification that needs to be considered. Depending on which downstream processes are present in the system, such as cryogenic recovery of liquids, or wherefor the treated gas is being used, such as for pipeline or residential use, the required discharge specification can differ from the requirements of the sub-quality gas .
As the concentrations of CO2 and H2S in the raw inlet gas and the allowable acid gas levels in the final product can vary substantially, no single AGR process is superior in all circumstances, and consequently, many processes are presently in use. CO2 and H2S removal can be a single step or two-step process.
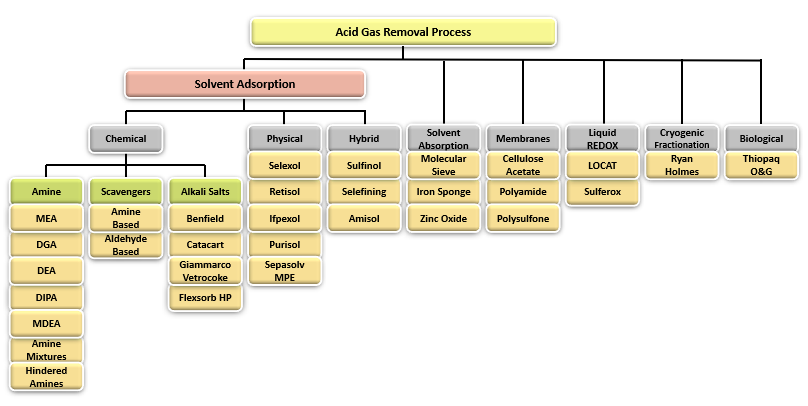
Acid Gas Removal Processes
Fig. 2 shows a simple chart summarizing the most important Acid Gas Removal processes and groups them into generally accepted categories. Some of the most widely used technologies are Amine Adsorption, Hybrid Systems and Membranes.
Figure 2 - Acid Gas Removal Processes
Some of the most important items that must be considered before a process is selected include:
- The type and concentration of impurities and hydrocarbon composition of the gas.
- The temperature and pressure at which sour gas is available
- The outlet gas specification
- The volume of gas to be processed
- Specifications for the residue gas, the acid gas, and liquid products
- The selectivity required for the acid gas removal
- The capital, operating, and royalty costs for the process, and….
- Local environmental constraints.
If gas sweetening is required offshore, both size and weight are additional factors that must be considered. Where CO2 and H2S removal is performed offshore, it is prudent to understand that the acid gas from the sweetening system needs to be either flared or re-injected back to the reservoir depending on the customer requirements.
Selection criteria can be complex; Fig. 3 presents a general overview of technology selection based on feed and outlet acid gas concentrations.
Figure 3 - Selection Matrix for Acid Gas Removal Processes
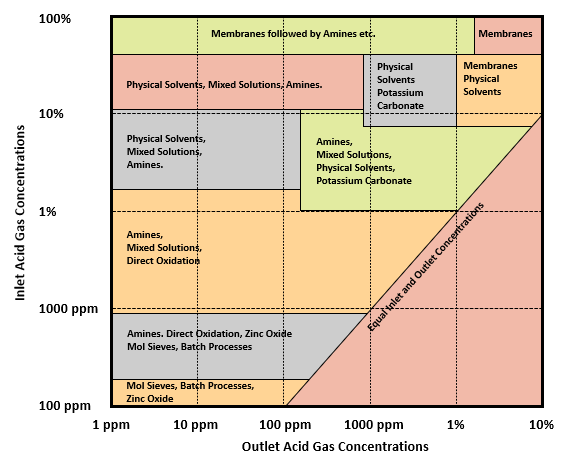
For example, removing CO2 from the feed stream containing 75 mol% CO2 down to 2 mol% CO2 in the product, membranes followed by amine absorption technology may fit well in the process. For selectively removing small amounts of H2S, mostly less than 100 ppm by volume, scavenger or molecular sieve technology will be ideal.
Bulk Acid Gas Separation Processes - Membrane Technology
Removing all the acid gases in a single processing stage is not always practical nor economical. Gas streams that contain significant quantities of acid gases often undergo bulk removal of those contaminants down to a level at which the more conventional acid gas processing methods become viable. In such cases, membrane systems are the most common technology used (Fig. 3).
Figure 4 - Membrane CO2 Removal Process Flow Diagram[2]
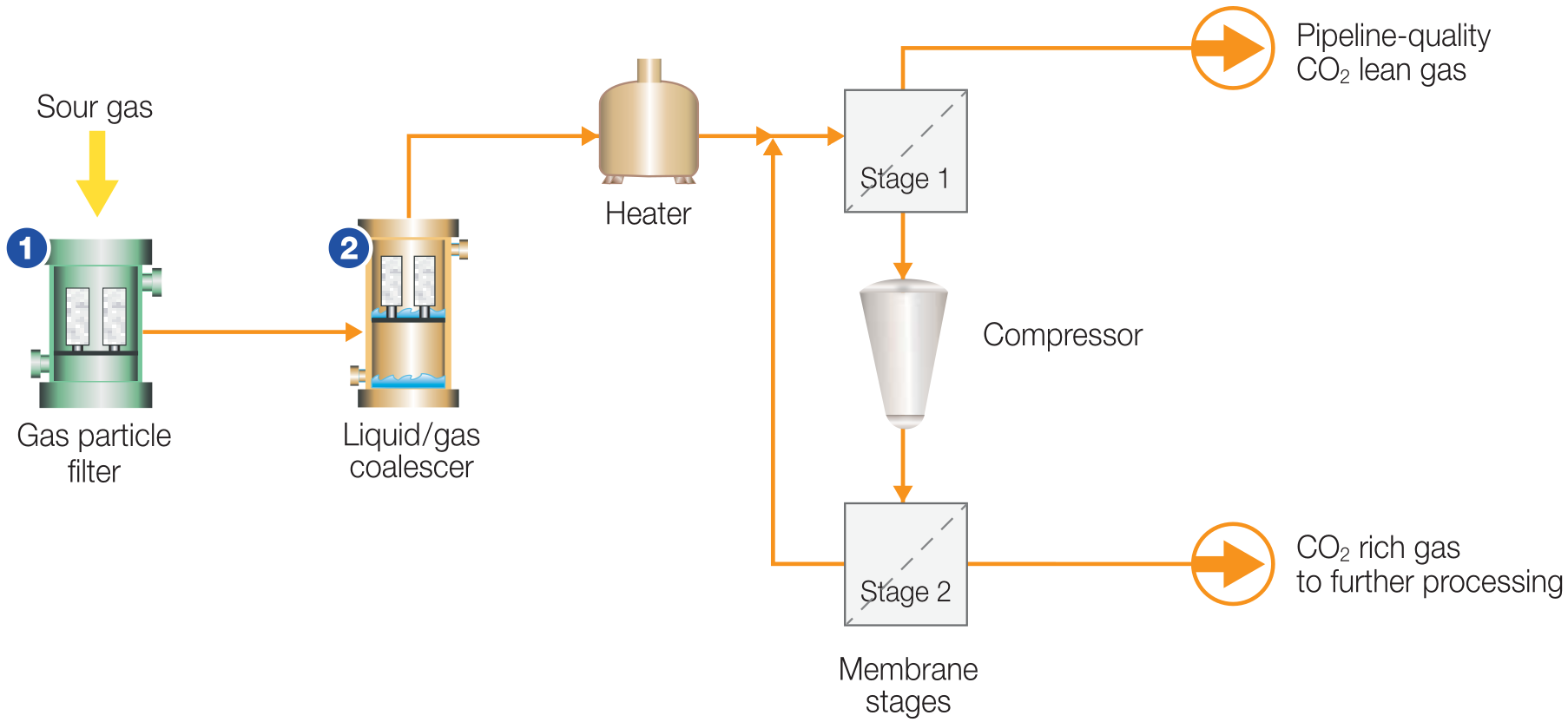
(1) Membrane element protection: Gas particle filters to control contaminant ingression into
membrane separation unit, (2) Membrane protection: Liquid/gas coalescers to remove
incoming liquids
Gas treating membrane systems provide a safe and efficient option for bulk removal of water vapor and carbon dioxide from natural gas, and are an ideal choice for removing CO2 offshore where there is a strict limitation on equipment footprint and weight. Membrane systems are extremely adaptable to various gas volumes, CO2 concentrations, and/or product-gas specifications. They can efficiently process feed gas containing 15-90 mol% CO2 to the required CO2 specification in the product gas. When a natural gas stream containing CO2 is fed to a membrane, the CO2 will permeate the membrane at a faster rate than the natural gas components. In this way, the feed stream will be separated into a CO2-rich, low pressure permeated stream and a CO2-depleted, high-pressure retained natural gas stream. Membrane technology is very well proven with a track record of processing feed streams larger than 750 MMSCFD.
Acid Gas Membranes are made from a variety of polymers including polyamides, polyimides, and most commonly cellulose acetates, the selection of which will impact performance. Membranes for Bulk Acid Gas Removal fall into two main types: Hollow Fibre, and Spiral Wound.
Bulk H2S Removal Processes - Amine Sweetening
Whilst Membranes are presently the preferred method for dealing with large volumes of CO2, their use in handling H2S is limited, especially when the quantity of H2S is large. When dealing with large quantities of H2S, Amine Absorption remains the dominant technology, although there is significant development work being undertaken with membranes (Fig. 5).
Figure 5 - Amine Cricuit Simplified Process Flow Diagram[3]
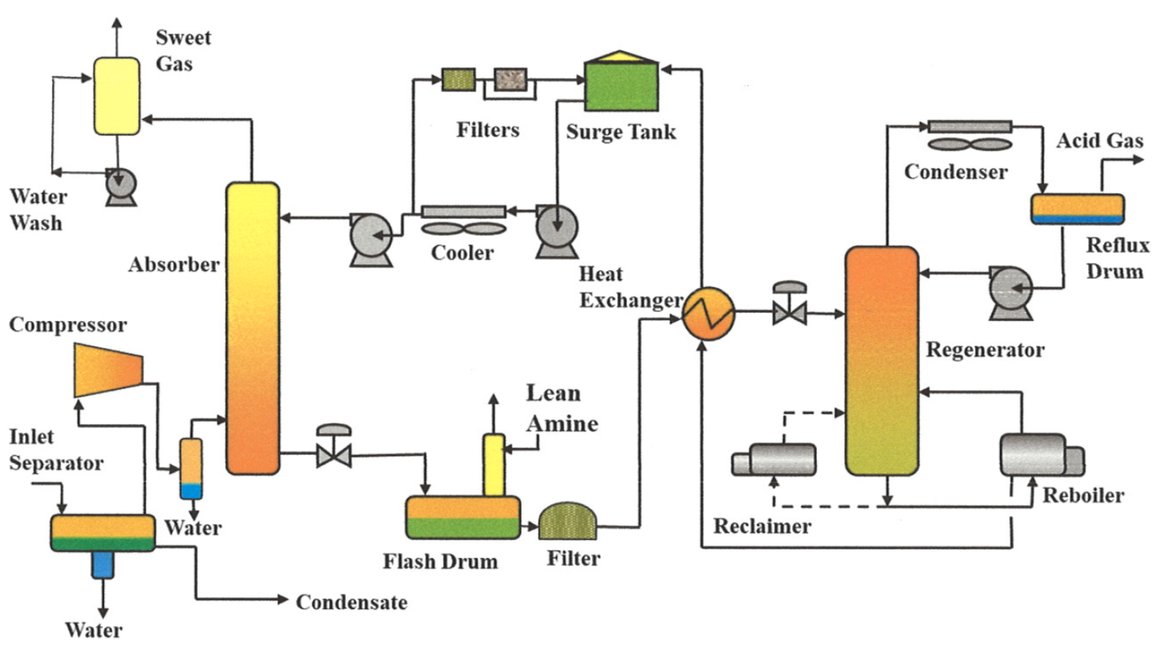
A typical Amine Unit consists of an absorber (contactor) and regenerator (stripper) along with their ancillary equipment. A process gas stream is first passed through the absorber, where H2S and CO2 are removed by the “lean” solvent. The now “rich” solvent is sent to the regenerator where the acid gases are removed and either sent to flare or a Sulfur Recovery Unit (SRU).
The are many desirable characteristics for a sweetening (Amine) solvent such as:
- Meeting the required removal of H2S and other sulphur compounds
- The pickup of hydrocarbons must be low.
- The acid gas pickup per unit of solvent circulated must be high.
- Selective removal of acid gases is desirable, and
- The solvent should be cheap and readily available.
Unfortunately, there is no one solvent that has all the desirable characteristics. This makes it necessary to select from the various solvents that are available the solvent that is best suited for treating the particular sour gas mixture, considering that such mixtures vary in H2S and CO2 content and ratio, in content of heavy or aromatic compounds, and in content of COS, CS2 and mercaptans.
There are essentially three different types of amine solutions currently available, based on their chemical formula and reactivity:
- Primary Amines – MEA & DGA:
Primary amines provide the lowest hydrocarbon adsorption. However, they require significant heat for regeneration due to their stronger bonding with acid-gas anions.
- Secondary Amines – DEA & DIPA:
Secondary amines, like DEA, have lower heats of reaction with H2S and CO2, are less corrosive, and can be used in higher concentrations leading to higher acid gas loadings. This results in a reduced circulation rate and in lower capital and operating costs.
- Tertiary Amines – MDEA:
Tertiary amines, like MDEA, are an ideal choice for lower losses of solvent and provide selectivity for H2S over CO2 when contacting gas streams containing both acid gases.
While most of the sour gas is sweetened with regenerative solvents, for slightly sour gas with a lower H2S content, it may be more economical to use an alternate method such as scavenger solvents or solid agents.
Alkali Salts Process
The hot potassium carbonate process for removing CO2 and H2S was developed back in the 1950’s. Although the process was originally developed to remove CO2, it can also remove H2S if it is present along with CO2. The process is similar in concept to the amine process in that the acid gases are physically absorbed and then react chemically with the solution being recirculated. However, special designs are required if the H2S levels are to reach pipeline specifications, or the CO2 needs to be reduced to low levels.
Physical Solvent Absorption
There are a number of different physical absorption processes available in the market for removal of CO2 and H2S. Unlike the Amine and Alkali salt processes, the absorption of the acidic contaminants H2S, CO2 and COS is essentially by chemical means but the presence of the physical solvent increases the physical solubility in the solvent of these contaminants thus improving the efficiency of the absorption process. The physical solubility of certain contaminants such as Mercaptans and Carbonyl Sulphide is also increased to such an extent that these contaminants can be removed to low levels.
In general, the economics of CO2 recovery is strongly influenced by the partial pressure of CO2 in the feed gas. At low partial pressures, physical solvents are not advisable because the compression of the gas for physical absorption is expensive. The concentration of heavy hydrocarbons in the feed gas greatly affects the choice of gas treating solvent. If the concentration of heavy hydrocarbons is high, a physical solvent may not be the best option due to higher co-absorption of hydrocarbons, particularly butanes plus. Unlike natural gases where hydrocarbon co-absorption can be a problem for physical solvents, synthesis gases do not contain appreciable quantities of hydrocarbons. This makes physical solvents particularly applicable to synthesis gas treating.
- Absorption processes are generally most efficient when partial pressures of the acid gases are relatively high, as this is the driving force for absorption.
- The solvents used have a strong affinity for heavy hydrocarbons, so the processes are most efficient when these species are present in low concentrations.
- Solvents can be chosen for selective removal of sulphur compounds, allowing the CO2 to pass through.
- Energy requirements for Regeneration in absorption systems are lower than for systems where there is a chemical reaction involved.
- Separation can occur at near ambient temperature
- Partial dehydration occurs along with removal of the acid gases, whereas Amine system produce water saturated gas that must be dehydrated.
Physical Adsorption
Acid gases, as well as water can be effectively removed by physical adsorption onto an adsorption media. Applications are limited because water displaces the acid gases from the adsorbent bed. Even at ambient temperatures and low partial pressures, substantial quantities of both acid gases are absorbed.
Molecular sieves can be used to reduce H2S levels down to the levels required for residential or industrial use (6 milligrams H2S per standard cubic meter of gas), but this requires a very high bed regeneration temperature (600°F or 315°C) for an extended time, and can come with the potential for hazardous Carbonyl Sulphide (COS) formation.
Cryogenic Fractionation
Cryogenic fractionation is a distillation process that at first glance seems a good prospect for removing CO2 and H2S form natural gas, because the vapor pressures are considerably different.
However, one can come across challenges in separating the following components:
- CO2 from Methane (the CO2 can freeze when it becomes concentrated)
- CO2 from Ethane (as both components form an azeotrope, and hence complete separation by simple distillation is impossible), and
- CO2 from H2S (the mixture has a pinch point at high CO2 concentrations making distillation very difficult)
An alternative way is to separate on the basis of phase separation. The mixture of acid gases along with the other components of natural gas is cooled to semi-cryogenic temperatures, whereby the contaminant condenses partially and after which the condensate is separated. Several processes have been developed based on this principle and there seems to be a continuous interest in the industry to further enhance the use of cryogenic liquefaction of acid gases.
The energy consumption of these processes is limited and makes them useful for high contaminant levels. The drawback is the large size of the installations and capital costs involved in the process as compared to conventional acid gas treatment processes.
Non-Regenerable Hydrogen Sulphide Scavengers
When the quantity of sulphur to be removed is small (lower than 1000 lbs of sulphur per day removal capacity), many of the previous processes mentioned become uneconomical, and small-scale batch processes are used for H2S removal. These processes typically use a non-regenerable scavenger, which fall into two categories: Solid Based and Liquid based.
The Solids based processes use Iron or Zinc Oxides as the scavenger, whilst the liquid processes employ one of the following:
- Nitrites
- Amine-Aldehyde Condensates
- Caustic Scrubbing
- Aldehydes
- Oxidizers, or
- Metal-oxide Slurries
Disposal of the hazardous spent scavenger is always an important consideration before selecting this option.
Biological Processes
There is also the option to utilize a biological process for the removal of H2S. Called the “Thiopaq” Process, this option can also be used for sulphur recovery.
References
- Description adapted from: Markus Sprenkel, 15th June 2020, A Discussion on Acid Gas Removal and Handling in Natural Gas Treatment and Processing, Linkedin
- Pall Corporation, Membrane CO2 Removal
- 3. Sulfur Recovery Engineering, Amine Process