Hydroprocessed (or hydrodewaxed) base oils are high‑purity paraffinic base oils produced entirely through catalytic hydrogen‑based processes rather than solvent refining. They are obtained by severe hydrotreating and/or hydrocracking of vacuum gas oils or deasphalted oils, followed by catalytic hydroisomerization dewaxing and final hydrofinishing. In these steps, sulfur, nitrogen, metals, and nearly all aromatics are removed, while straight‑chain wax molecules are isomerized into branched isoparaffins that provide high viscosity index and low pour points instead of being removed as slack wax.
The resulting hydroprocessed base oils are typically water‑white, 99+% saturated, very low in sulfur, and exhibit high viscosity index (often ≥115), low volatility, excellent oxidation and thermal stability, and very good low‑temperature fluidity.
Hydroprocessed (hydrodewaxed) base oils sit mainly in Group II, Group II+, Group III and Group III+ as defined by API, because the all‑hydroprocessing route gives them very high saturates, very low sulfur, and high viscosity index. They typically have densities of about 0.84–0.88 g/cm³ at 15°C, depending on viscosity grade and degree of hydroprocessing.
Figure 1 - Conversion of feed molecules vs. relative hydrocracking severity [10]
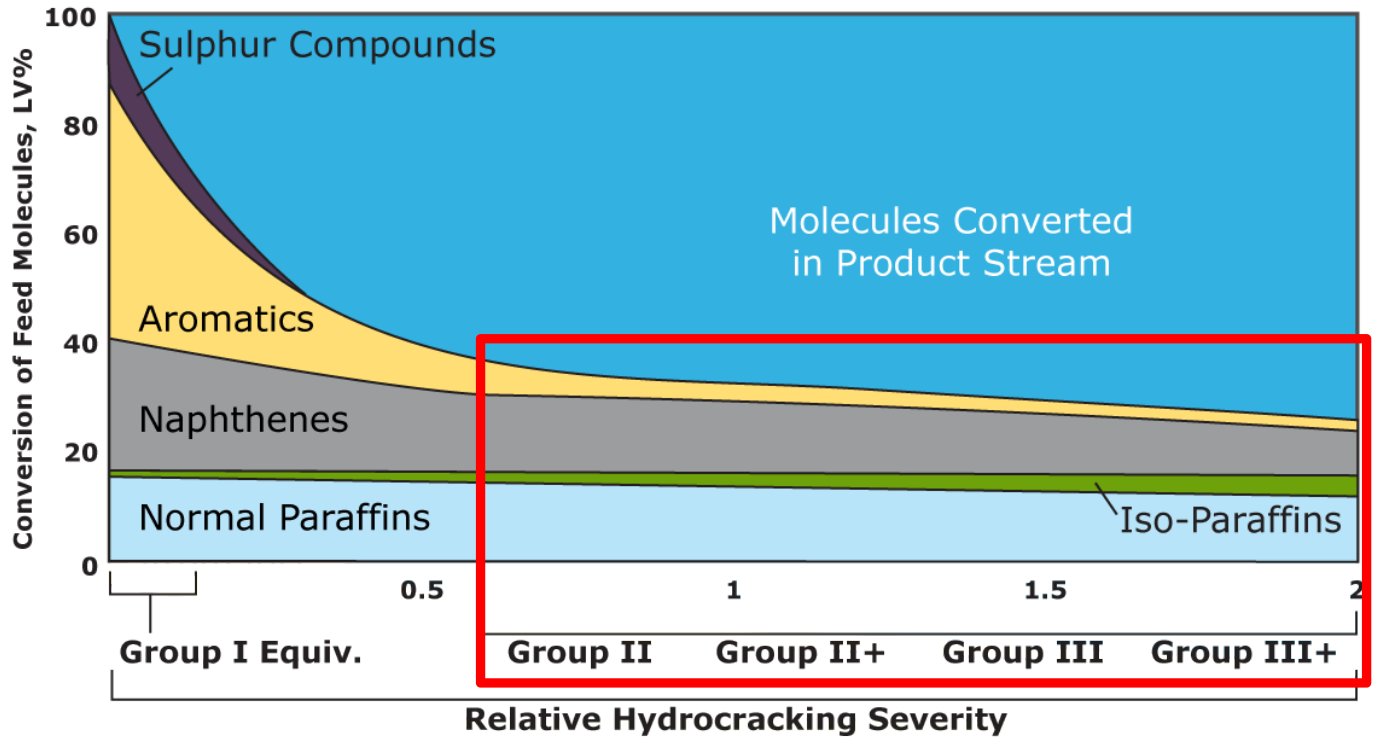
Residual normal paraffins are converted to iso-paraffins during catalytic dewaxing.
Residual aromatics are converted to iso-naphthenes during hydrofinishing.
Comparative characteristics and properties of hydrodewaxed base oils, SN base oils and bright stock are provided in the following table:
| Feature |
Solvent Neutral (SN) Base Oils |
Hydroprocessed (Hydrodewaxed) Base Oils |
Bright Stock |
Typical
process
route |
Solvent extraction + solvent dewaxing (no hydrofinishing) |
Hydrotreating / hydrocracking + catalytic isomerization dewaxing + hydrofinishing |
Deasphalting
+ solvent refining
+ hydrofinishing |
Feedstock
origin |
Vacuum distillates |
Vacuum distillates or DAO / hydrocracker unconverted oil |
Deasphalted
vacuum residue |
Viscosity
range
100°C) |
Light to heavy neutrals
(~4–14 cSt) |
Light to heavy neutrals (~4–14+ cSt, higher VI) |
Very high
(~26–35 cSt) |
Aromatics
content |
Moderate (good solvency) |
Very low
(high saturation) |
Low–moderate, heavier molecules |
Sulfur
content |
Higher
(typical Group I) |
Very low to near-zero (Group II/III range) |
Low (hydrofinished residue) |
Viscosity
ndex |
Moderate
(≈90–105) |
High (≈110–140+ depending on severity) |
Moderate (for very viscous grades) |
Color/
appearance |
Pale to amber |
Water‑white,
very clear |
Bright, pale colored heavy oil |
Oxidation
/thermal
stability |
Good |
Very good
to excellent |
Good for
high‑viscosity applications |
Low‑
temperature properties |
Improved by solvent dewaxing, limited vs hydrodewaxed |
Excellent pour point
and CCS from isomerization dewaxing |
Poor; mainly used where low‑temp
flow is less critical |
| Solvency |
High (favours additive and seal compatibility) |
Lower than SN
(may need co‑solvent stocks/additives) |
High solvency
for heavy‑duty applications |
Typical
ses |
Cost‑effective automotive
and industrial lubricants |
Premium engine / industrial oils,
low‑SAPs, long‑drain, high‑performance blends |
Viscosity builder in gear oils, marine oils, industrial and engine oils requiring very high viscosity |
References
- Mascherpa (December 22, 2022). Hydrogenation process of base oils
- Petro‐Canada Lubricants Inc.. Base Oil Hydrotreating Process
- Wright J. (June 27, 2012). The Fundamentals of Mineral Base Oil Refining. Machine Lubrication
- Eser S. FSC 432: Petroleum Processing — Processing and Conversion of Vacuum Distillation Residue. PennState - College of Earth and Mineral Sciences
- Outhwaite A. & Rosenbaum, J. (2011). Base oils – An evolving landscape. Lubrisense White Paper, 11-11. Axel Christiernsson AB
- Sing Group (October 30, 2024). Group III & III+ (Base Oils)
- DUTM B.V.. Base Oils
- Lubrex. API Base Oil Groups I, II & III: Classification, Key Properties & Comparison Table
- Mahmodi B., Black Water Petrochemical FZE (Dec 30, 2024). The Science Behind Base Oils: Chemistry and Classification