Catalytic dewaxing, commonly known as Hydroisomerization dewaxing or isomerization dewaxing, is an advanced catalytic process that converts long-chain normal paraffins (n-paraffins) into branched isoparaffins to reduce the pour point and improve low-temperature flow properties of lubricating base oils and middle distillates. Unlike conventional catalytic cracking-based dewaxing or solvent dewaxing, which physically remove waxy components, hydroisomerization selectively reshapes wax molecules into valuable base oil components, thereby maximizing product yield and quality.
Historical Development
Traditional lube base oil production relied on solvent dewaxing—a physical separation process developed in the early-to-mid 20th century that removed waxy n-paraffins by refrigeration and filtration. While effective at reducing pour point, solvent dewaxing suffered from low yields (typically 70-80%) due to wax rejection and high capital and operating costs associated with solvent recovery and refrigeration systems.
The first generation of catalytic dewaxing emerged in the 1970s-1980s using shape-selective zeolite catalysts that selectively cracked n-paraffins to lighter hydrocarbons. While this improved economics, it still resulted in yield loss through conversion to fuel-range products rather than preserving lube-range molecules.?
The breakthrough came in 1993 when Chevron commercialized its ISODEWAXING® technology at the Richmond, California refinery, marking the beginning of modern hydroisomerization dewaxing. This technology represented a paradigm shift: instead of removing or cracking waxy components, it isomerized them into high-quality, low-pour-point isoparaffins, achieving yields of 95-98% while producing superior Group II and Group III base oils. Following Chevron's success, ExxonMobil commercialized its MSDW™ (Mobil Selective DeWaxing) process, and other licensors developed competing technologies.
Process Chemistry and Fundamentals
Reaction Mechanisms
Hydroisomerization dewaxing involves two primary reaction pathways occurring simultaneously over bifunctional catalysts:
Figure 1 - Chemistry of catalytic dewaxing inside molecular sieving zeolites
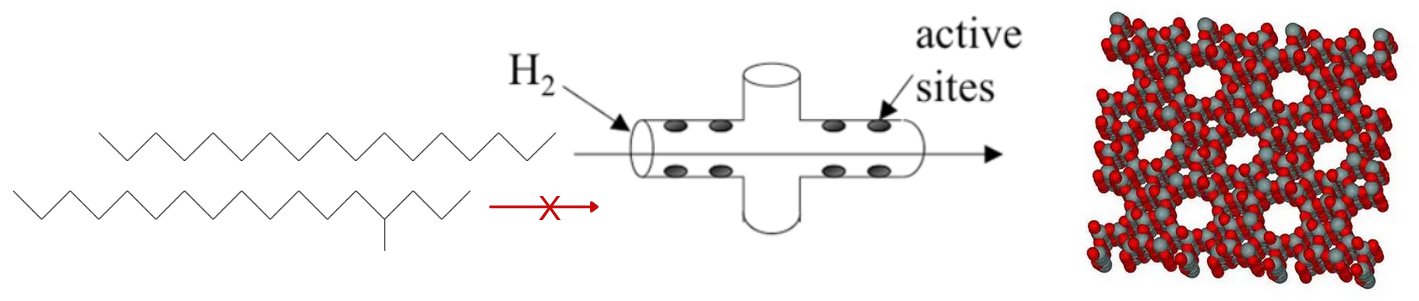
Selective cracking takes place inside the pores of the catalyst.
n-Alkanes can enter the pores, but bulky iso-alkanes cannot.
Zeolite structure shown on the right hand side.
- Hydroisomerization (Primary Reaction)
- Long-chain n-paraffins (>C15) are isomerized into mono- and multi-branched isoparaffins
- Wax molecules (C20-C50) are converted to branched structures with significantly lower pour points
- Isomerization preserves carbon number while changing molecular geometry
- Branched isoparaffins exhibit high viscosity index (VI), low pour point, and excellent oxidation stability
- Mild Hydrocracking (Secondary Reaction)
- Limited cracking of some long-chain paraffins to lighter hydrocarbons
- Selective hydrocracking produces valuable diesel and naphtha by-products
- Catalyst selectivity is tuned to minimize cracking and maximize isomerization
- Aromatic Saturation
- Concurrent hydrogenation of aromatics to naphthenes
- Improves oxidation stability and reduces aromatic content to meet Group II/III specifications
Catalyst Systems
Modern hydroisomerization catalysts are bifunctional systems containing:
Acidic Function: Shape-selective zeolite molecular sieves
- ZSM-5 (MFI structure): Early catalyst generation
- ZSM-22 (TON structure): Preferred for lube dewaxing due to unidimensional pore structure?
- Beta zeolite: Used for slack wax processing
- SAPO-11: Silico-aluminophosphate molecular sieves for enhanced selectivity
- Pore sizes (5-7 Å) allow n-paraffins to enter while excluding bulky isoparaffins and aromatics?
Metallic Function: Noble metal components for hydrogenation/dehydrogenation
- Platinum (Pt): Most common, typically 0.05-0.5 wt%?
- Palladium (Pd): Alternative or combination with Pt
- Dispersed on supports (alumina, silica-alumina)
Modern catalyst formulations employ graded catalyst beds with progressively increasing zeolite modification (dealumination, silicon insertion) to optimize selectivity and activity distribution along the reactor.
Detailed Process Description
Feed Preparation Section
Feedstock: Hydrotreated or hydrocracked vacuum gas oil (VGO), deasphalted oil (DAO), unconverted oil (UCO), solvent-extracted raffinates, or slack wax.
Feed pretreatment requirements:
- Sulfur: <50 ppm (preferably <10 ppm) to prevent catalyst poisoning
- Nitrogen: <5 ppm (noble metal catalysts are nitrogen-sensitive)?
- Aromatics: Pre-saturated in upstream hydrotreating if necessary
- Water: Dried to prevent hydrolysis of zeolite structure
Hydrogen preparation:
- High-purity hydrogen (>95%) from makeup and recycle streams
- Compressed to reaction pressure: 500-2000 psig (3.4-13.8 MPa)
- Preheated to 600-750°F (315-400°C) through heat exchange with reactor effluent
Feed-hydrogen mixing:
- Feed is heated to reaction temperature through heat exchangers
- Combined with hydrogen at H2/oil ratios of 2000-8000 SCF/bbl (350-1400 Nm³/m³)
- Higher ratios improve catalyst stability and product quality
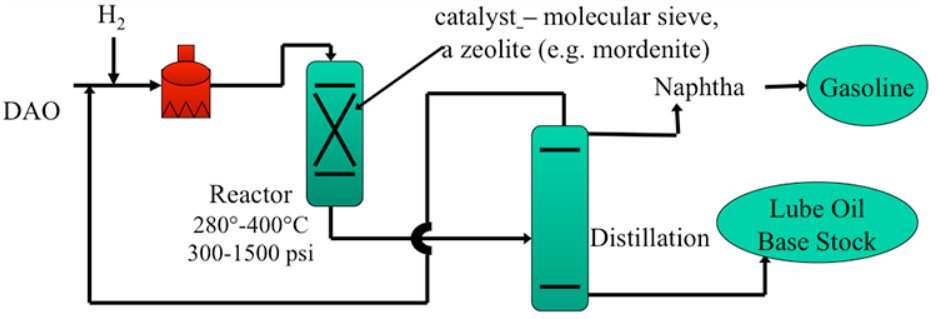
Figure 2 - Simplified catalytic dewaxing process flow diagram [3]
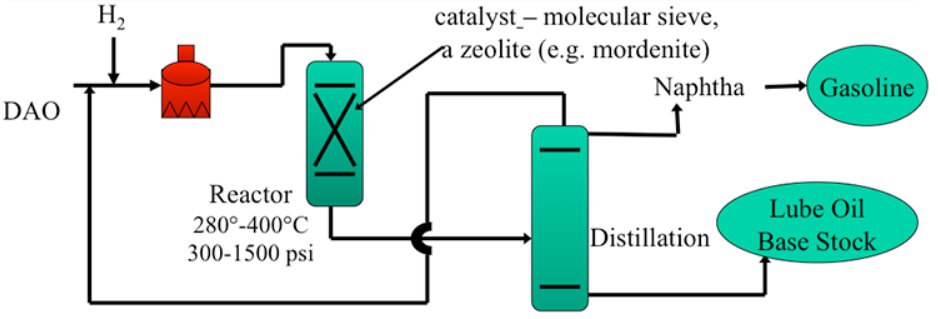
Hydroisomerization Reactor Section
Reactor configuration:
- Fixed-bed, downflow reactors (typically 1-3 reactors in series)
- Catalyst volumes: 50-300 m³ depending on capacity
- Multiple catalyst beds with inter-stage quench systems to control exothermic reactions
Operating conditions:
- Temperature: 575-750°F (300-400°C)
- Light neutral oils: 575-650°F (300-345°C)
- Heavy neutral/bright stock: 650-750°F (345-400°C)
- Milder than hydrocracking: 390-650°F for optimal isomerization selectivity
- Pressure: 500-2000 psig (3.4-13.8 MPa), typically 800-1200 psig
- LHSV (Liquid Hourly Space Velocity): 0.5-2.0 hr?¹
- Lower LHSV for heavier feeds and lower target pour points
- Hydrogen partial pressure: 400-1500 psi
Temperature control:
- Cold hydrogen or liquid recycle quench between catalyst beds?
- Maintains optimal temperature profiles to prevent catalyst deactivation
- Exothermic heat release: ~50-150 BTU/lb feed
Product pour point control:
- Achieved by adjusting temperature and space velocity
- Pour point range: -9°C to below -40°C (ultra-low pour point grades)?
- Independent control of VI and pour point through catalyst selection and conditions
Product Separation and Finishing
Hot high-pressure separator:
- Operating at reactor pressure and 600-700°F
- Separates hydrogen-rich gas from liquid product
- Hydrogen recycled after amine treating to remove H2S
Cold low-pressure separator:
- Reduces pressure to 50-150 psig
- Temperature: 100-150°F
- Flashes light ends (C1-C4) and naphtha
Fractionation section:
- Atmospheric distillation tower separates products:
Hydrofinishing (optional):
- Final mild hydrotreating at 450-600°F, 300-800 psig
- Improves color, oxidation stability, and aromatics saturation
- e.g. CLG ISOFINISHING technology for Group II/III specifications
Process Performance and Economics
Yield Comparison
| Process |
Base Oil Yield |
Pour Point |
VI Range |
| Solvent Dewaxing |
70-80%? |
-9 to -15°C |
90-100 |
| Conventional Catalytic Dewaxing |
75-85% |
-9 to -18°C |
95-105 |
| Hydroisomerization Dewaxing |
95-98%? |
-9 to <-40°C |
95-140+ |
Technical Advantages
Product quality:
- Higher VI products (120-140+ achievable) vs. solvent dewaxing (90-100)
- Ultra-low pour points (<-40°C) vs. solvent dewaxing (-15°C typical)
- Excellent oxidation stability due to paraffinic composition
- Low aromatics content (<5% for Group II, <1% for Group III)
Yield benefits:
- 15-25% higher base oil yield than solvent dewaxing
- Valuable diesel by-product with low pour point and high cetane
- Can process 100% wax feeds (slack wax, Fischer-Tropsch wax) without recycle
Operational flexibility:
- Process both lube oil feedstocks and diesel fuels
- Broad range of feedstocks: light neutral through bright stock
- Independent control of VI, pour point, and volatility?
Environmental advantages:
- No solvent losses or emissions
- Lower energy consumption (no refrigeration)
- Cleaner products with lower sulfur and nitrogen
Economic Comparison
Capital costs:
- Hydroisomerization: Medium (USD 100-200 million for 15,000 bpd unit)
- Solvent dewaxing: Medium-High (refrigeration and solvent recovery systems)
- Overall capital comparable, but favors hydroisomerization for grassroots projects?
Operating costs:
- Hydroisomerization: Lower due to higher yields and no solvent costs
- Catalyst replacement: 3-5 years typical life (MSDW: >12 years reported)?
- Energy: Hydrogen consumption 200-600 SCF/bbl vs. refrigeration energy for solvent dewaxing
- Maintenance: Lower for hydroisomerization (no rotating equipment, filters, or refrigeration)
Economics favor hydroisomerization when:
- Producing Group II/III base oils (higher value products)
- Processing heavier feeds where yield advantage is maximized
- Integrating with hydrocracking or hydrotreating units (shared hydrogen systems)
Major Licensed Processes
- Chevron Lummus Global (CLG) - ISODEWAXING®
- ExxonMobil - MSDW™ (Mobil Selective DeWaxing)
- Shell - XHVI (Xtra-High Viscosity Index)
- Sinopec - Proprietary Hydroisomerization
Summary
Hydroisomerization dewaxing represents the state-of-the-art in base oil production, having displaced solvent dewaxing as the preferred technology for Group II and Group III base oils since the 1990s. By isomerizing rather than removing waxy components, the process achieves superior yields (95-98%), product quality (VI up to 140+), and operational flexibility while maintaining competitive capital and lower operating costs. The dominance of Chevron's ISODEWAXING and ExxonMobil's MSDW technologies in the global market reflects the maturity and proven performance of hydroisomerization dewaxing as the cornerstone of modern base oil manufacturing.
References
- ScienceDirect Topics. Hydroisomerisation Reaction.
- Buffalini M.H. (June 22, 2014). Solvent vs. Catalytic Dewaxing.
- Eser S. FSC 432: Petroleum Refining — Catalytic Dewaxing. Penn State University.
- Chevron Lummus Global, ISODEWAXING.
- Chevron Lummus Global, All-Hydroprocessing.
- ExxonMobil, Lubricant base stock dewaxing (MSDW™ technology).
- Jack (June 25, 2019). Catalytic Dewaxing Process by ExxonMobil. Oil & Gas Process Engineering.
- Guan-Dao L. et al. (Nov. 2008). All Hydroprocessing Route to High Quality Lubricant Base Oil Manufacture Using Chevron ISODEWAXING Technology. Paper presented at AIChE 2008 Annual Meeting.
- Apelian M.R. et al. US Patent 5,885,438A, Wax hydroisomerization process. (March 28, 1996: Application filed by Mobil Oil AS).
- Liming J. et al. CN Patent 101942320B, Method for producing base oil by isodewaxing (July 9, 2009: Application filed by China Petroleum and Chemical Corp, Sinopec Fushun Research Institute of Petroleum and Petrochemicals).