Alkylation Process in the Refinery
Since crude oil generally contains only 10-40% of hydrocarbon constituents in the gasoline range, refineres typically use an FCCU to convert high molecular weight hydrocarbons into smaller and more volatile compounds, which are then converted into liquid gasoline-size hydrocarbons. Byproducts of the FCC process also creates other low molecular-weight alkenes and iso-paraffin molecules which are not desirable.[1]
Alkylation transforms these byproducts into larger iso-paraffin molecules or alkylate, a high-octane gasoline blending component. Alkylate product is a mixture of branched paraffinic hydrocarbons of gasoline boiling range (mostly isoheptane and isooctane). Alkylate has a Motor Octane (MON) of 90-95 and a Research Octane (RON) of 93-98. Because of its high Octane and low vapor pressure, alkylate is considered an excellent blending component for gasoline.[2],[3]
In the refinery, isobutane is alkylated with isobutene in the presence of a strong acid catalyst, either sulfuric acid or hydrofluoric acid. Depending on the acid used, the process is referred to as a SAAU or a HFAU. However, oil refinery employees may simply refer to the unit as the Alkyl or Alky Unit. Propylene present in the feed in small concentration also reacts with isobutane to form isoheptane.[2],[3]
Figure 1 - Principal Alkylation Reactions[4]
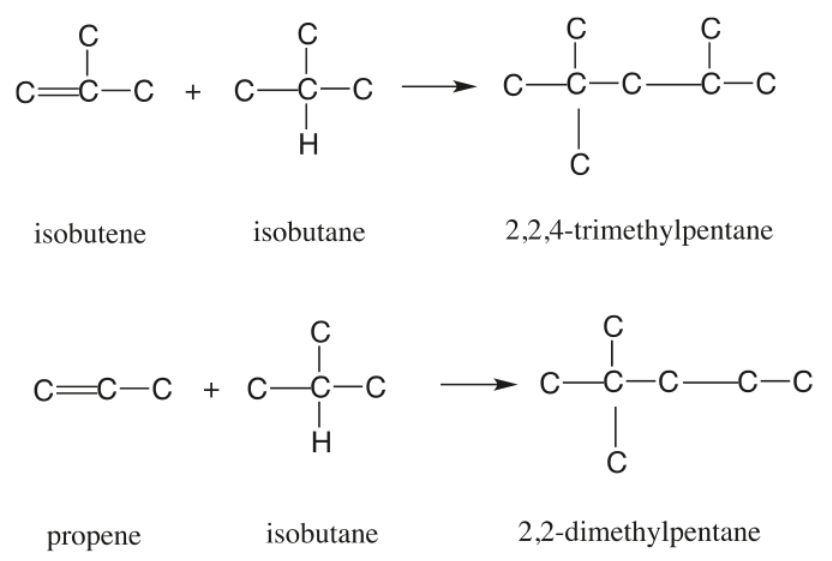
Under reaction conditions unfavorable to alkylate formation, propylene may also polymerize to form the undesirable product polypropylene. Amylenes can undergo a similar reaction to form alkylate, but since amylenes have a high octane number to start with, their conversion to alkylate is not as advantageous, as in the case of butylenes. Another side Reaction that can negatively affect the formation of alkylate is ester formation by the reaction of olefins with sulfuric acid.[2]
General Process Description
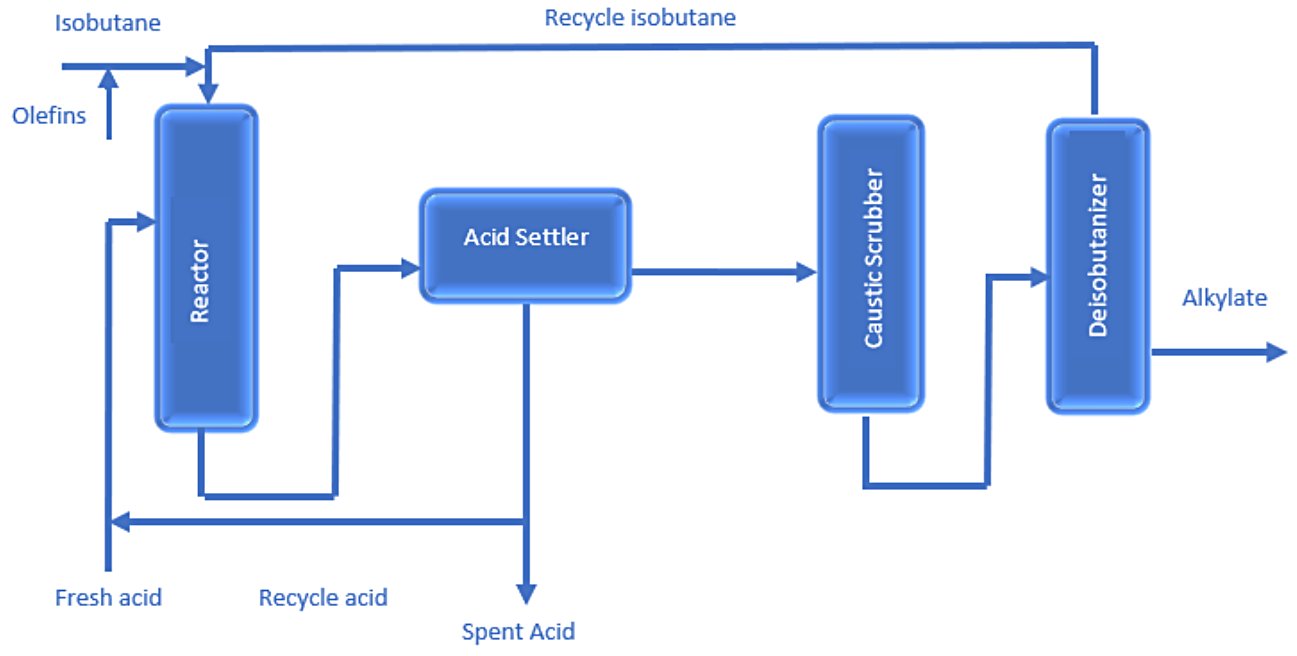
The general Process Description of the Alkylation Unit is as follows (Fig. 2):[5]
- Prior to entering the reaction section, the olefin and isobutane feeds are treated in a coalescer to remove water, sulfur, and other contaminants.
- A mixture of light olefins and isobutane (either C4s, C3s, or a mix) is injected into a reactor with a series of reaction chambers. These reaction chambers are filled with an acid catalyst; either sulfuric acid (H2SO4) or hydrofluoric acid (HF). Due to the presence of a catalyst, isobutane reacts with an olefin to produce either a C7 or C8 naphtha-range product called alkylate.
- The reactor effluent goes to a settler, in which hydrocarbons separate from the acid. The acid is separated and returned to the reactor. The hydrocarbons are washed with caustic and sent to a fractionator.
- The fractionation section comprises a depropanizer, a deisobutanizer, and a debutanizer. Alkylate from the deisobutanizer can go directly to motor-fuel blending, or it can be reprocessed to produce aviation-grade gasoline.
Figure 2 - Simplified Alkylation Process Block Flow Diagram.[5]
Conventional alkylation processes employing liquid mineral acids, such as hydrofluoric acid or sulfuric acid, pose significant safety and environmental risks. Solid acid catalysts offer a promising alternative, replacing liquid acids and eliminating many of these concerns.[6] Ionic liquids are also used in place of the older generation of strong Bronsted acids[1] with the same motives.
H2SO4 Alkylation Process Description
Olefins (C3-C4) from the FCC Unit are combined with isobutane (i-C4). Concentrated sulfuric acid (H2SO4) is then mixed in and fed to the reactor that is operated with refrigeration to minimize the formation of by-products. The alkylation reaction takes place, combining the olefins with the isobutane to form the alkylate product. The hydrocarbons leaving the reactor mostly consist of alkylate, unreacted isobutane, n-butane and propane.[7]
The acid and alkylate products are separated by a settler and the acid is recovered for re-use in the reactor. The reactor hydrocarbons are separated by the main fractionator where the isobutane is recycled to the reactor, Alkylate is drawn from the bottom, and an n-butane product stream is separated from a side stream.[7]
The light ends of the process fluids are used in the refrigeration loop and a side stream is passed through a propane stripper to recover propane. Effective separation is a critical component of an efficient, reliable, and safe sulfuric acid alkylation process.[7]
Figure 3 - Representative Sulfuric Alkylation Process.[7]
HF Alkylation Process
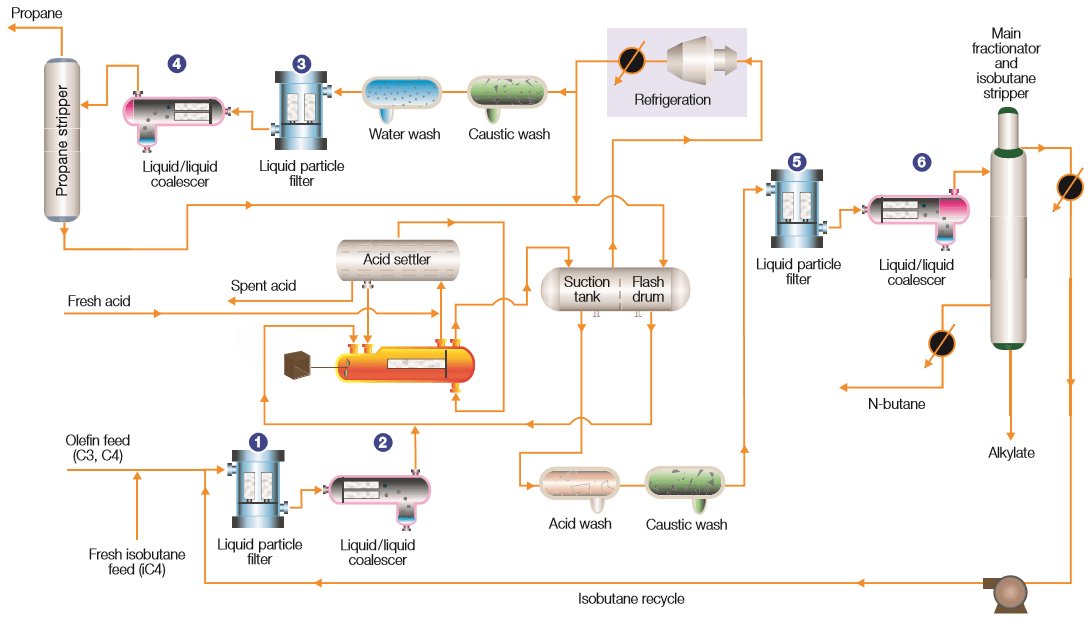
In the hydrofluoric acid alkylation process the HF catalyst protonates the alkenes (propylene, butylene) to produce reactive carbocations, which alkylate isobutane. The reaction is carried out at mild temperatures (0 and 30 °C) in a two-phase reaction as described below (Fig. 4)[8]:
Olefins (C3-C4) from the FCC unit are combined with isobutane (i-C4) and are first treated with a mol sieve bed to remove water. Concentrated HF is then mixed in and fed to the reactor. The alkylation reaction is a result of combining the olefins with the isobutane to form the alkylate product consisting of high octane value products which are later blended into the gasoline pool.[9]
The HF and alkylate product are separated by a settler and the HF is recovered for re-use in the reactor. The reactor hydrocarbons are separated by the main fractionator where the isobutane is recycled to the reactor, alkylate is drawn off the bottom and an n-butane product stream is separated and treated with caustic (KOH).[9]
Most of the overheads consist of HF acid fumes and propane gas. They are then cooled and the HF is separated. Propane is further treated through the propane splitter to remove any dissolved HF and finally treated with alumina to remove fluorinated organics, and then caustic (KOH) to neutralize any remaining acid. Effective separation is a critical component of an efficient, reliable and safe H2SO4 alkylation process.[9]
Figure 4 - Representative Hydrofluoric Acid Alkylation Process.[9]

Solid Acid Alkylation
In Solid Acid Alkylation, an acidic active site within the porous matrix of a catalyst fixed bed will promote the reaction between an isoparaffin and an olefin to yield a high-octane product. The catalyst matrix can also promote side reactions, just like the liquid acid process to produce undesired, high molecular weight polymer species. These polymer species reduce octane number and increase end point of the alkylate product. The main difference between this technology and sulfuric acid alkylation is the catalyst is made up of a fixed bed of porous pellets, typically a zeolite with an active species impregnated into the crystalline structure, to catalyze the alkylation reaction.[10]
Ionic Liquid Alkylation Technology
Ionic liquid alkylation technology is a novel process for producing high-octane motor fuels, claimed to offer a safer and more environmentally friendly alternative to traditional technologies using hydrofluoric (HF) or sulfuric acids as liquid alkylation catalysts.[11]
The key features of the technology are: :
- Ionic Liquid Catalyst:
The technology employs a non-aqueous liquid salt, or ionic liquid, as a catalyst at temperatures below 100°C. This eliminates the volatility and hazardous properties associated with conventional acids.
- Wider Feedstock Range:
Ionic liquid alkylation can process a broader range of feedstocks, including heavier and more complex molecules, than traditional acid-based technologies.
- Lower Catalyst Volume:
The ionic liquid catalyst requires less volume than conventional acid catalysts, reducing the overall equipment size and costs.
- Easier Handling:
The ionic liquid is non-corrosive and non-hazardous, making it easier to handle and transport compared to HF or sulfuric acids.
- Higher Product Yield and Selectivity:
Ionic liquid alkylation technology has been shown to produce high-quality alkylate products with high yields and selectivity.
Commercial Technologies
The first alkylation units entered in service in 1940. In 2009 around 1,600,000 barrels per day of capacity were installed worldwide, with an equal share of 800,000 barrels per day for SAAU and HFAU technologies.[1]
Hydrofluoric Acid Catalyst Alkylation Technologies
Sulfuric Acid Catalyst Alkylation Technologies
Ionic Liquid Catalyst Alkylation Technologies
Solid Acid Catalyst Alkylation Technologies
References
- Alkylation Unit, Wikipedia.
- Alkylation, Brewiki.
- REFINING - ALKYLATION, techSTAR.
- Dr. Semih Eser © Penn State, FSC 432 PETROLEUM PROCESSING, Alkylation, image licensed under CC BY-NC-SA 4.0.
- Nasir Hussain, 19th Sep 2022, Alkylation Process In Petroleum Refinery, The Petrosolutions.
- Prompt: 'Solid Acid Catalyst Alkylation', 9th Nov 2024, Brave search summarizer.
- H2SO4 Alkylation Process Description, PALL.
- Hydrofluoric Acid, Wikipedia.
- HF Alkylation Process Description, PALL.
- S. Zhang et al., 9th Sep 2016, Alkylation Technology Study FINAL REPORT, NORTON engineering.
- Prompt: 'Ionic Liquid Alkylation Technology', 9th Nov 2024, Brave search summarizer.