Process History and Technology Variants
Technology Origins
Low-temperature methanol wash is a physical acid gas removal (AGR) process that uses refrigerated methanol as a solvent to separate acid gases—primarily CO₂ and H₂S—from industrial gas streams at subzero temperatures. The technology was pioneered in the 1950s when Linde Aktiengesellschaft (Munich, Germany) and Lurgi Mineralöltechnik (Frankfurt, Germany) developed this technology marketed under the Rectisol® trademark.
The fundamental principle—cold methanol physical absorption at -40°C to -75°C operating temperatures—has since become the basis for both proprietary licensed technologies and non-proprietary generic implementations worldwide.
Proprietary Rectisol® Technologies
Rectisol® remains a registered trademark held by the original technology developers and their successors:
Linde plc (formed from the 2018 merger of Linde AG and Praxair) continues to independently own, license, and market its own Rectisol® technology.
Air Liquide Global E&C Solutions acquired what was originally "Lurgi's Rectisol®" technology in 2007 when Air Liquide purchased the Lurgi engineering firm from GEA Group for approximately €550 million. Air Liquide now licenses its own Rectisol® technology and holds the associated trademark rights through its German subsidiary.
While both licensed Rectisol® versions share the same fundamental principle, they are not identical technologies. EPA documentation from the 1980s notes that "the basic processes are similar but with some significant differences" in process configuration, equipment design, and proprietary optimizations. Each licensor offers distinct engineering approaches and process enhancements developed over their independent 70-year technology evolution.
Generic Low-Temperature Methanol Wash Variants
Since methanol is a non-proprietary, inexpensive, and readily available solvent, numerous generic variants of low-temperature methanol wash have been developed, particularly in China for coal gasification applications. These non-licensed implementations use the same fundamental cold methanol physical absorption principle but feature:
- Different process configurations and equipment arrangements
- Varied pressure levels and flash regeneration sequences
- Alternative heat integration schemes
- Customized designs for specific feedstocks and applications
Other Trademarked Methanol-Based Processes
Ifpexol® (licensed by IFP Energies Nouvelles/Axens) is the only other trademarked methanol-based cold absorption process, though it was developed primarily for natural gas treatment with an integrated two-stage configuration combining dehydration and acid gas removal.
Process Summary and Chemistry
Fundamental Principle
Low-temperature methanol wash is a physical acid gas removal (AGR) process that uses methanol (CH₃OH) as a solvent at subzero temperatures (typically -40°C to -75°C) to selectively absorb acid gases from synthesis gas and other industrial gas streams. Unlike amine-based chemical absorption processes, this technology relies on physical solubility governed by Henry's Law, where gas solubility increases with decreasing temperature and increasing pressure.
The process operates at high pressure (typically 2.76 to 6.89 MPa or 400 to 1000 psia) using non-proprietary, inexpensive, and readily available methanol as the absorption medium. This combination of low temperature and high pressure creates highly favorable conditions for acid gas absorption.
Key Chemistry
The process employs physical absorption rather than chemical reaction. The solubility of acid gas components in cold methanol follows the order:
- H₂S > COS > CO₂ > CO > H₂ > N₂
This differential solubility enables selective removal of contaminants, with the process capable of achieving:
- Total sulfur removal to <0.1 ppm (including H₂S and COS)
- CO₂ removal to ppm levels
- H₂S purification down to 50 ppbv in treated syngas
- CO₂ product purity of 97-98.5 mol% suitable for carbon capture and storage (CCS) or enhanced oil recovery (EOR) applications
The process also removes trace contaminants including NH₃, HCN, CS₂, mercaptans, BTX aromatics, and metal carbonyls. This comprehensive contaminant removal capability makes it particularly suitable for syngas from coal or heavy hydrocarbon gasification, which typically contains these impurities.
Selective Separation Capability
Low-temperature methanol wash is particularly valued in syngas-based applications from gasification because it can selectively separate H₂S and CO₂ into two distinct product streams. This selective removal is achieved through multi-stage flash regeneration at different pressure levels, exploiting the differential volatility of absorbed components. The capability enables:
This flexibility in process configuration allows independent management and valorization of each acid gas component, making the technology highly adaptable to different downstream process requirements and environmental constraints.
Detailed Step-by-Step Technology Description
Process Configuration
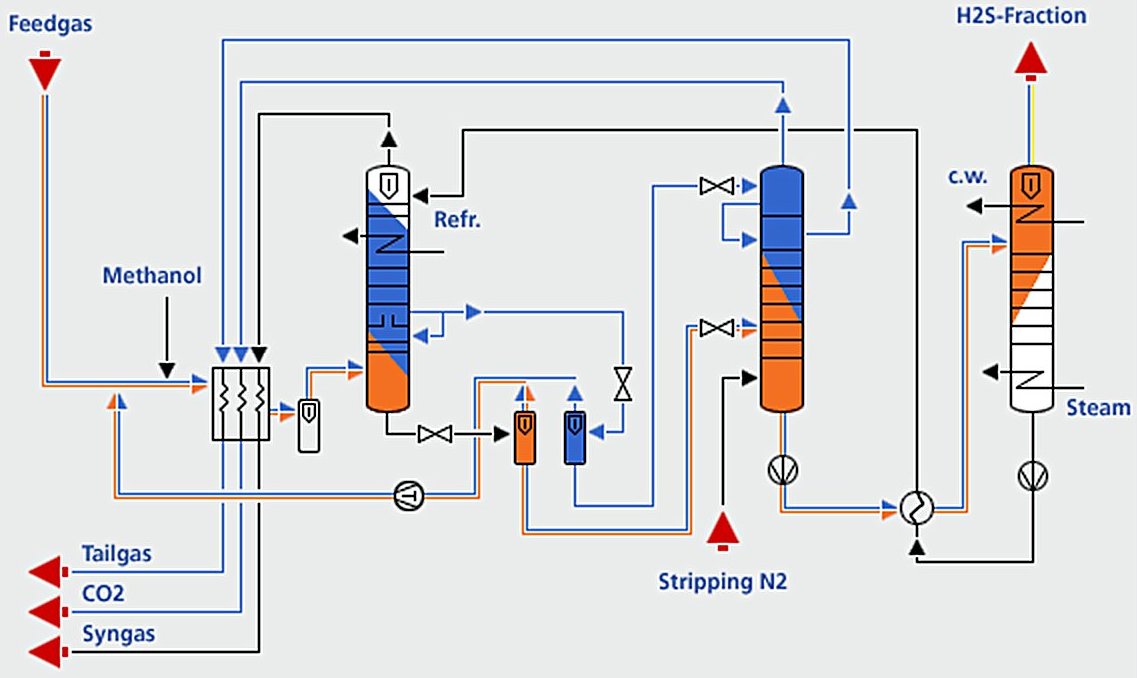
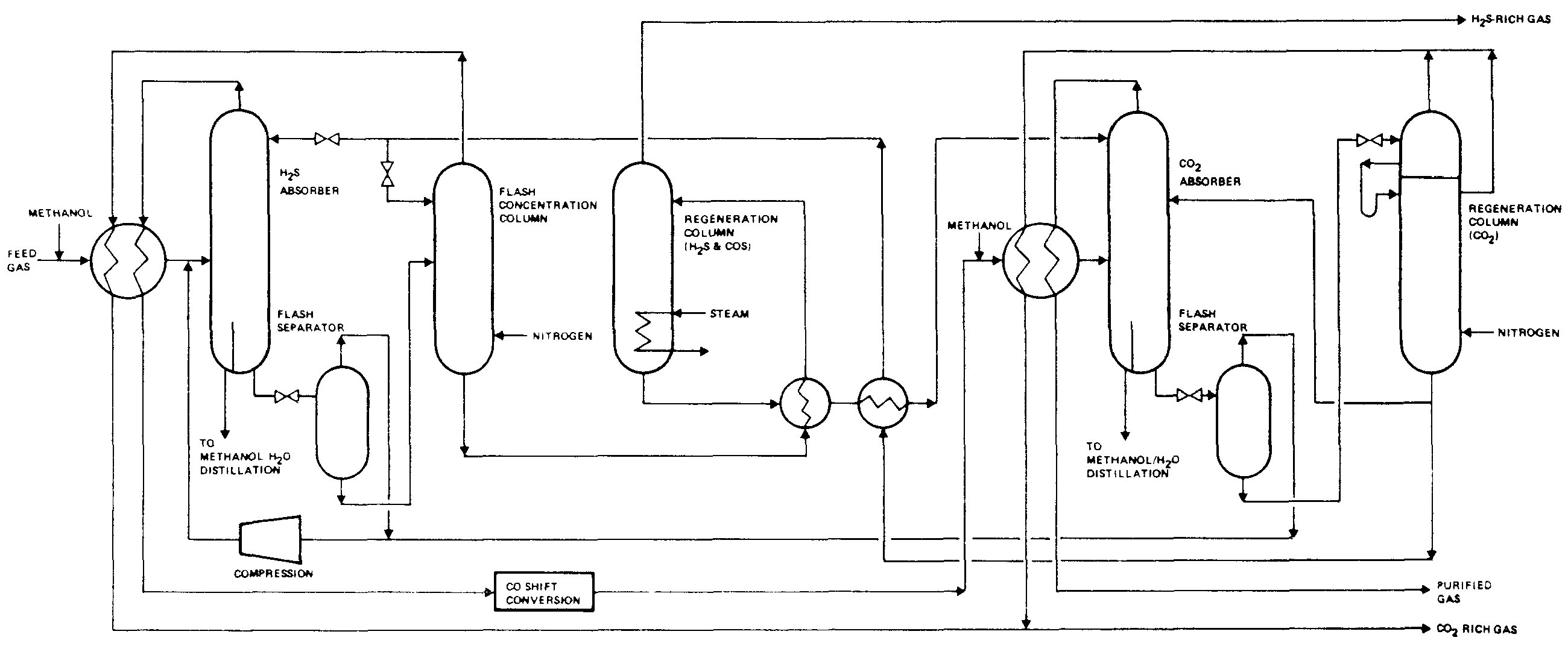
Low-temperature methanol wash consists of four integrated sections. The description below is based on a representative configuration, though specific implementations vary depending on licensor, application requirements, and engineering preferences (see ref. nr. 11 for various configurations)
Modderfontein Selective Rectisol Section[11]
1. Acid Gas Absorption Section (H₂S and CO₂ Removal)
- Main Absorber Column: the absorber is the primary unit where acid gases are physically absorbed into cold methanol solvent. Typical design parameters include:
- Feed conditions: Raw syngas typically enters at 30-40°C and 30-60 bar
- Number of theoretical stages: 15-25 depending on purity requirements and feed composition
- Operating temperature: -40°C to -75°C in different column sections
- Operating pressure: 35-60 bar (2.76-6.89 MPa)
- Multi-section absorber configuration (from bottom to top):
- H₂S/COS removal section (bottom stages): Preferential absorption of sulfur compounds due to higher solubility
- CO₂ bulk removal section (middle stages): Main CO₂ absorption zone
- CO₂ cooling section: Temperature control for optimal absorption
- CO₂ main wash section: Further CO₂ removal
- CO₂ fine wash section (top stages): Final polishing to achieve ultra-low acid gas concentrations
- Process flow:
- Raw syngas enters the absorber containing typical acid gas concentrations from coal gasification (20-30% CO₂, 0.5-2% H₂S, plus CO, H₂, N₂, and trace contaminants). Regenerated cold methanol solvent is introduced at the column top at temperatures between -40°C and -55°C and pressures of 55-65 bar.
- The absorber includes intercoolers (side coolers or intermediate cooling stages) to remove the heat of absorption and maintain optimal absorption temperatures throughout the column. These coolers are critical for process efficiency as physical absorption is exothermic and temperature-sensitive. Typical cooling duty ranges from 2-5 MW per cooling stage depending on gas throughput.
- Solvent withdrawal: Methanol-rich streams are withdrawn at different points:
- Bottom stream: Contains highest concentration of H₂S and dissolved CO₂ (typically 65-75% methanol, 25-35% acid gases)
- Side stream(s): May be withdrawn at intermediate points for selective regeneration (typically 70-75% methanol, 20-30% CO₂, lower H₂S content)
- Clean syngas product exits the absorber top at cold temperatures (-40°C to -50°C) and high pressure (typically 55-60 bar) with composition achieving:
- CO₂: <0.01-0.1 mol% (depending on specification)
- H₂S: <0.1-1 ppmv (or 50-100 ppbv for ultra-low sulfur applications)
- Overall H₂S removal efficiency: >99.9%
2. CO and H₂ Recovery Section
- Flash Drum System: Physical absorption inevitably dissolves valuable syngas components (CO, H₂) along with acid gases. A flash recovery system recovers these components to improve process economics. This recovery step is essential for maintaining high overall CO and H₂ yields, typically recovering >95% of valuable syngas components.
- Operating principles:
- Rich solvent streams are expanded through pressure reduction valves from absorber pressure (55-60 bar) to intermediate pressure (20-25 bar)
- Flash drums separate vapor and liquid phases at the reduced pressure
- Flash vapor streams recover dissolved CO and H₂ along with some CO₂ (typical composition: 60-85% CO₂, 10-20% CO, 5-15% H₂, minor H₂S)
- Vapor streams are compressed (with isentropic efficiencies of 80-85%) back to absorber feed pressure and recycled to the absorber inlet via a mixer
- Liquid phases proceed to CO₂ desorption (containing 70-75% methanol, 25-30% CO₂, minor H₂S)
3. CO₂ Desorption and Recovery Section
- Multi-stage Flash and Column System: The CO₂ desorption section exploits the differential volatility of absorbed components to produce high-purity CO₂ while recovering methanol solvent. Configurations vary but typically include:
- Low-Pressure Desorption Column:
- Column type: Absorber/stripper with or without reboiler
- Number of stages: 12-20
- Operating pressure: 2-6 bar
- Rich solvent is expanded through multiple pressure reduction valves from intermediate pressure (20-25 bar) to low pressure (2-6 bar)
- Flash evaporation releases CO₂ while H₂S (more soluble) tends to remain in liquid phase
- Overhead vapor: High-purity CO₂ (typically 98-99.6% CO₂) at -40°C to -55°C and 2-6 bar
- Coolers maintain low temperatures (-40°C to -45°C) to condense methanol vapors and return as reflux
- High-Pressure Desorption Column (optional, depending on configuration):
- Number of stages: 15-25
- Operating pressure: 6-20 bar
- Processes different rich solvent stream or intermediate regeneration products
- Overhead vapor: CO₂ product (95-99% purity) at -30°C to -40°C
- Multi-stage flash drums: Bottom liquid from desorption columns may pass through successive flash separators (typically 2-4 stages) at progressively lower pressures to maximize CO₂ recovery and auto-refrigeration.
- Combined CO₂ product stream is compressed and typically achieves:
- Purity: 97-98.5% CO₂ (dry basis, CCS-ready)
- Delivery pressure: 80-150 bar for pipeline transport or storage
- Minor components: Residual methanol vapor, trace H₂S (<5 ppmv), inerts
- Reabsorber Column (for H₂S concentration):
- Processes flash vapors from low-pressure regeneration stages
- Uses lean methanol or nitrogen stripping to remove residual CO₂
- Re-absorbs flashed sulfur compounds (H₂S, COS) for routing to hot regeneration
- Enables separation of H₂S from CO₂ into distinct product streams
4. Methanol Solvent Regeneration Section
- Hot Regeneration and Solvent Recycling: Complete methanol regeneration requires thermal treatment to strip dissolved H₂S and residual CO₂, producing pure solvent for recycle.
- Distillation Column:
- Column type: Conventional distillation with partial condenser
- Number of stages: 8-12
- Operating pressure: Near atmospheric (1-1.5 bar)
- Methanol from CO₂ desorption columns is expanded to atmospheric pressure through expansion valve
- Reboiler duty: Provided by low-pressure steam (2-10 bar saturated steam) or waste heat recovery
- Overhead vapor distillate: H₂S-rich acid gas stream (typical composition: 15-25% H₂S, 75-85% CO₂) at atmospheric pressure
- H₂S/CO₂ ratio designed to meet Claus unit feed requirements (typically >1:2 to 1:3)
- Sent to sulfur recovery unit for H₂S conversion to elemental sulfur
- Bottom product: Regenerated methanol (>99.9% purity) at 60-75°C and atmospheric pressure
- Two-stage regeneration (optional configuration for energy optimization):
- First stage at moderate pressure (2-6 bar) for preliminary stripping
- Second stage at atmospheric pressure for final purification
- Reduces overall reboiler steam consumption by 15-20%
- Methanol Recycle System:
- Regenerated methanol is cooled in heat exchangers to near-ambient temperature (15-25°C)
- Pumped to absorber operating pressure (55-65 bar) using high-pressure centrifugal pumps (typical isentropic efficiency: 75-80%)
- Refrigerated to target absorption temperature (-40°C to -55°C) using refrigeration system
- Recycled to absorber column top
- Methanol make-up: Fresh methanol is continuously added to compensate for losses:
- Solvent losses: 0.04-0.1% of circulation rate due to methanol vapor pressure and mechanical losses
- Losses increase at higher operating temperatures
- Make-up typically ranges from 0.1-0.5 t/h per 400 t/h syngas throughput
5. Refrigeration System
- External Refrigeration (Critical Utility): Since physical absorption in methanol is highly temperature-dependent, substantial refrigeration capacity is required:
- Refrigeration cycle configuration:
- Cascade ammonia-ethane system for multi-level cooling
- Evaporation levels: Typically 2-4 temperature levels (-10°C, -30°C, -50°C, -70°C) depending on process requirements
- Compressor efficiency: Polytropic efficiency 80-84% for refrigeration compressors
- Major refrigeration loads:
- Cooling regenerated methanol from ambient to absorption temperature (-40°C to -55°C)
- Intercooling in absorber column (removing heat of absorption)
- Condensing methanol vapors in desorption column overheads
- Auto-refrigeration integration: Some process variants recover refrigeration duty by flashing high-pressure liquid streams and using the cooling effect to reduce external refrigeration requirements by 10-20%.
Key Process Parameters Summary
| Parameter |
Typical Range |
Unit |
| Absorber pressure |
35-60 |
bar |
| Absorber temperature |
-40 to -75 |
°C |
| Methanol feed temperature |
-40 to -55 |
°C |
| CO₂ desorption pressure |
2-20 |
bar |
| Final regeneration pressure |
1-1.5 |
bar |
| Compressor isentropic efficiency |
80-85 |
% |
| Pump isentropic efficiency |
75-80 |
% |
| Expander isentropic efficiency |
85-90 |
% |
| Methanol circulation rate |
100-200 |
kg/kg acid gas |
| Refrigeration temperature levels |
-10 to -70 |
°C |
Process Flexibility and Efficiency
Process Configurational Flexibility
Low-temperature methanol wash is a highly flexible technology that can be configured in multiple ways depending on application requirements, downstream process needs, and environmental constraints:
Acid Gas Removal Modes:
- Selective H₂S removal: CO₂ remains in treated gas while H₂S is removed to <0.1 ppm; suitable when CO₂ is desired in product gas (e.g., for H₂/CO ratio adjustment in synthesis applications)
- Selective CO₂ removal: Bulk CO₂ removal with minimal H₂S co-absorption; used when separate H₂S treatment is preferred
- Dual removal with separate recovery: Both acid gases removed with H₂S and CO₂ recovered as distinct product streams—the most common configuration for coal gasification
- Total acid gas removal: Combined acid gas stream produced for disposal or single-stage treatment
- Partial CO₂ removal: Controlled CO₂ removal to adjust CO₂/H₂ ratio for downstream synthesis requirements (Fischer-Tropsch, methanol)
Downstream Application Adaptability: The process can be tailored to produce syngas meeting specifications for:
- Hydrogen production (including blue hydrogen with CCS)
- Ammonia synthesis gas
- Methanol synthesis gas
- Fischer-Tropsch synthesis
- Synthetic Natural Gas (SNG) production
- Oxogas/oxochemicals production
- Power generation (IGCC applications)
- Pure CO production
Product Stream Flexibility:
- Clean syngas: Purity specification adjustable from <1 ppmv to <0.1 ppmv total sulfur depending on downstream catalyst requirements
- CO₂ product: Delivery conditions adjustable from low-pressure vent gas to high-pressure supercritical CO₂ (>150 bar) for CCS or EOR
- H₂S-rich stream: H₂S/CO₂ ratio optimized for sulfur recovery unit requirements (Claus or WSA process)
This configurational flexibility makes low-temperature methanol wash particularly suitable for complex gasification-based process schemes where multiple products are needed simultaneously, such as combined production of hydrogen, CO, fuel gas, and chemical feedstocks.
Removal Efficiency and Product Yields
Acid Gas Removal Performance (typical achievable levels):
| Component |
Feed
Concentration |
Product
Concentration |
Removal
Efficiency |
| H₂S |
0.5-2 mol% |
<0.1-1 ppmv (50-100 ppbv) |
>99.95% |
| COS |
50-500 ppmv |
<10 ppbv |
>99.9% |
| Total Sulfur |
1-2 mol% |
<0.1 ppm |
>99.99% |
| CO₂ |
20-35 mol% |
<0.01-0.1 mol% |
70-99%* |
*CO₂ capture level depends on process configuration and optimization objectives
Reference Case Performance (from detailed simulation):
- Raw syngas feed: 28% CO₂, 1.3% H₂S, 23.4% CO, 46.9% H₂, 0.4% N₂
- Clean syngas product: <0.01% CO₂, <0.6 ppmv H₂S (50 ppbv)
- H₂S removal: >99.95% (from 1.3 mol% to 50 ppbv)
- CO₂ capture: 96.4% (from 28 mol% to <0.01 mol%)
Trace Contaminant Removal:
- NH₃ (ammonia): >99% removal
- HCN (hydrogen cyanide): >99% removal
- CS₂ (carbon disulfide): >95% removal
- Mercaptans: >99% removal
- BTX aromatics (benzene, toluene, xylene): >90% removal
- Metal carbonyls (Ni(CO)₄, Fe(CO)₅): >95% removal
- Mercury (Hg): Partial removal depending on speciation
Product Stream Purities:
- Clean syngas: CO₂ <0.01-0.1 mol%, H₂S <0.1-1 ppmv, total sulfur <0.1 ppm
- CO₂ product: 97-98.5% purity (dry basis, CCS-ready with <5 ppmv H₂S)
- H₂S-rich stream to sulfur recovery: H₂S/CO₂ molar ratio 1:2 to 1:5, typically 15-25% H₂S concentration
Syngas Component Recovery (valuable gas conservation):
- CO recovery: >99% (via flash recovery system)
- H₂ recovery: >98% (via flash recovery system)
- Overall carbon efficiency: >98%
Solvent Consumption and Losses
Methanol Consumption:
- Make-up requirement: 0.04-0.1% of total circulation rate
- Typical make-up for 400 t/h syngas throughput: 0.15-0.5 t/h methanol
- Primary loss mechanisms: Vapor losses due to methanol's relatively high vapor pressure, mechanical losses in pumps and seals, carryover in product gas streams
- Losses increase at higher operating temperatures (temperature-dependent vapor pressure)
Methanol circulation rates: Typically 100-200 kg methanol per kg acid gas removed, depending on operating temperature and pressure
Environmental Performance
Indirect CO₂ Emissions (from utility consumption): Process optimization with structured packings reduces indirect CO₂ emissions by 16% through lower steam and electricity consumption
Economic Performance / Commercial Experience
Capital Expenditure (CAPEX)
CAPEX Structure
- Absorber column: Represents approximately 10% of total process unit equipment costs
- High-pressure equipment: Thick-walled vessels required for 35-60 bar operation increase capital costs
- Refrigeration system: Represents 25-35% of total CAPEX; significant investment in compressors, heat exchangers, and refrigerant inventory
- Overall CAPEX: Higher than ambient-temperature physical solvent processes (e.g., Selexol) or chemical absorption (e.g., aMDEA) due to refrigeration requirements
CAPEX Optimization with Structured Packings (vs. traditional tray design):
- Absorber diameter reduction: 25-27%
- Absorber CAPEX reduction: 39% (structured packing absorber costs 61% of tray design)
- Overall process unit equipment CAPEX reduction: 7%
- Additional benefits: Lower pressure drop, higher liquid handling capacity
Operating Expenditure (OPEX)
Major operating cost components:
- Refrigeration energy: Largest single operating cost (40-50% of total OPEX)
- Compression power: Syngas compression, CO₂ compression, recycle gas compression (30-35% of OPEX)
- Steam consumption: Methanol regeneration reboiler duty (10-15% of OPEX)
- Electricity: Pumps, auxiliaries (5-10% of OPEX)
- Methanol make-up: Minimal (<2% of OPEX due to low solvent cost)
OPEX Reduction with Process Optimization: Structured packing implementation achieves 17% overall OPEX savings:
- Refrigeration duty reduction: 19%
- Electricity consumption reduction: 16%
- Steam consumption reduction: 18%
Energy Costs
Specific Energy Consumption:
- Non-optimized design: 755 kJ/kg CO₂ captured (specific exergy consumption)
- Optimized design: 662 kJ/kg CO₂ captured (12% improvement through systematic heat integration and process optimization)
- Energy breakdown: ~60% refrigeration, ~30% compression, ~10% thermal regeneration
CO₂ Capture Cost Sensitivity:
- Optimal CO₂ capture range: 90-98% (nearly linear cost increase)
- Beyond 98-99% capture: Exergy consumption increases dramatically due to finite CO₂ solubility in methanol
- Economic optimum typically at 95-97% CO₂ capture for CCS applications
Optimal Application Envelope
Low-temperature methanol wash is most economically attractive when:
- Feed pressure: >30 bar (physical absorption efficiency increases with pressure)
- Acid gas concentration: >20% total acid gases (high loading favors physical absorption)
- Purity requirements: Ultra-low sulfur specifications (<0.1-1 ppmv) required for downstream catalytic processes
- Feedstock: Coal, lignite, or heavy hydrocarbon gasification producing syngas with multiple contaminants
- Product requirements: Need for separate H₂S and CO₂ streams with high individual purities
- Integration opportunities: Synergy with cryogenic processes or where cold syngas product is beneficial
Commercial Track Record
Initial deployment: Low-temperature methanol wash technology was first deployed at large scale in the 1950s at Sasol's Fischer-Tropsch plant (Sasol One) in Sasolburg, South Africa, where three identical purification units with a combined capacity of 164 MMscfd were installed for coal gasification gas treatment using licensed Rectisol® technology. In 2004, the Sasol One site at Sasolburg converted from coal gasification to more efficient natural gas-based production processes. Sasol subsequently expanded operations with two larger facilities Sasol Two and Sasol Three at the Secunda CTL complex, each employing systems capable of treating over 1 billion standard cubic feet per day across four independent trains.
Licensed Rectisol® installations: Air Liquide (ex-Lurgi) reports >110 commercial Rectisol® references worldwide (including operating units, projects under construction, and completed engineering studies), with over 35 installations commissioned since 2005. Combined installations from both Linde plc and Air Liquide licensors exceed 85 operating units as of 2006, with current installations estimated at 100-120+ units.
Generic technology deployment: Beyond licensed Rectisol® units, numerous non-licensed generic low-temperature methanol wash installations have been deployed, particularly in China's coal chemical industry since the 2000s. These domestically-engineered implementations utilize the same fundamental cold methanol absorption principles for coal-to-methanol, coal-to-chemicals, and coal-to-SNG projects. The total global installation base (combining licensed and generic implementations) likely exceeds 200-250 operating units as of 2026.
Low-temperature methanol wash represents the benchmark acid gas removal technology for coal gasification applications globally, with particular concentration in South Africa (Sasol), China (coal-to-chemicals, largest deployment region), and North America (IGCC and synfuels projects).
References
- Air Liquide Engineering & Construction. Technology bulletin: Lurgi Rectisol™ (Document date: 2017)
- Wikipedia. Rectisol (Page edited: Mar 27, 2025)
- LaFond A.. Air Liquide Acquiring German Engineering Company Lurgi (Apr 17, 2007). Manufacturing.net
- Axens. IFPEXOL™
- Holcek, R.G. et al.. The IFPEX-1 process for natural gas dehydration/hydrate inhibition - the North American experience. Canada: N. p., 1996. Web
- Jack. Ifpexol Process by IFP Group Technologies (Jun 19, 2018). Oil & Gas Process Engineering
- Holmes M.. Summary of Dakota Gasification Company’s CO2 Capture and Transport, and Future Options for Gasification Systems (Jun 17, 2005). CSLF Mid-Year Meeting and Technology Workshop Regina, Saskatchewan, Canada
- Gatti M. et al.. Multi-objective optimization of a Rectisol® process (2014). DOI: 10.1016/B978-0-444-63455-9.50043-X
- Gamba, S. et al.. Acidic gas absorption by methanol: System modeling and simulation. In Proceedings of the International Conference on Applied Energy (ICAE 2012), Suzhou, China, July 5-8, 2012.
- U.S. Department of Energy (DOE), National Energy Technology Laboratory (NETL). Rectisol
- Ondich G.G.. United States Environmental Protection Agency (EPA). EPA-600/8-83-009: Control Technology Appendices for Pollution Control Technical Manuals (Apr 1983).
- Brixland S.. Master Thesis: Investigation of a CO-production of Substitute Natural Gas and Biomethanol Plant Compared to Stand Alone Biomethanol Plant (Apr 6, 2019). Lund University
- ScienceDirect. Rectisol Process
- Fives ProSim. ProSimPlus Application Example - Syngas Deacidification with Rectisol Process (Mar 2024)
- Schmidt, S. et al.. Rectisol™ Column Design with Structured Packings. Nitrogen+Syngas, no. 371 (May-June 2021): 44-47
- Nakamura T. & Senior C.L.. U.S. Department of Energy (DOE), Office of Scientific and Technical Information (OSTI). PSI-1356: Recovery and Sequestration of CO2 from Stationary Combustion Systems by Photosynthesis of Microalgae (Mar 2001)
- Enhance Energy Inc. and North West Redwater Partnership. Knowledge Sharing Report: Enhance Energy Inc. and North West Redwater Partnership (Mar 31, 2018)
- Ahrenfeldt, J., Jørgensen, B., & Thomsen, T.. Bio-SNG potential assessment: Denmark 2020 (Nov 2010). Danmarks Tekniske Universitet, Risø Nationallaboratoriet for Bæredygtig Energi. Denmark. Forskningscenter Risoe. RisoeR No. 1754(EN)
- 张述伟 et al. Chinese patent CN102806000A: Energy-saving one-step low-temperature methanol washing method. Sep 5, 2019: Application filed by Dalian Jiachun Gas Purification Technology Development Co ltd
- Koss U. et al.. United States patent US20110000366A1: Method for treating a process gas flow containing CO2. Sep 5, 2009: Application filed by Lurgi GmbH
- 亢万忠 et al.. Chinese patent CN1218866C: Low temperature methanol cleaning process. Apr 3, 2002: Application filed by China Petrochemical Corp, Sinopec Lanzhou Design Institute
- 张述伟 et al.. Chinese patent CN102078742B: Low-temperature methanol washing method for low-pressure raw gas. Nov 26, 2010: Application filed by Dalian University of Technology
- Jiang P.. PhD Thesis: Gasification-based clean conversion and utilization of coal, oil, shale and biomass (Jul 2020). University of Nottingham
- Sasol. Historical Milestones
- Global Energy Monitor Wiki. Sasol One power station
- Sasol. Operations / Locations
- Sparks S., University of Johannesburg. Between ‘artificial economics’ and ‘the discipline of market forces’: SASOL, from State Corporation to Privatisation (Aug 26, 2014)
- Encyclopedia.com. Sasol Limited (May 18, 2018)
- Meridian Economics. Transitioning SA's Petrochemical Value Chain
- Hoogendom, J.. OSTI ID: 5136590 - 17 years of gas production from coal. [SASOL] (Sep 1, 1972). Gas (Los Angeles), 48. U.S. Department of Energy (DOE), Office of Scientific and Technical Information (OSTI)
- Gert Sibande District Municipality. Sasol South Africa Limited - Secunda Operations (Feb 25, 2025). Government of South Africa