Ethanol dehydration is a catalytic process that converts ethanol into ethylene by acid-catalyzed removal of water molecules in fixed-bed reactors operating at elevated temperatures. The process represents an important pathway for producing renewable ethylene from bioethanol derived from sugarcane, corn, or other biomass feedstocks.
Reaction Chemistry and Thermodynamics
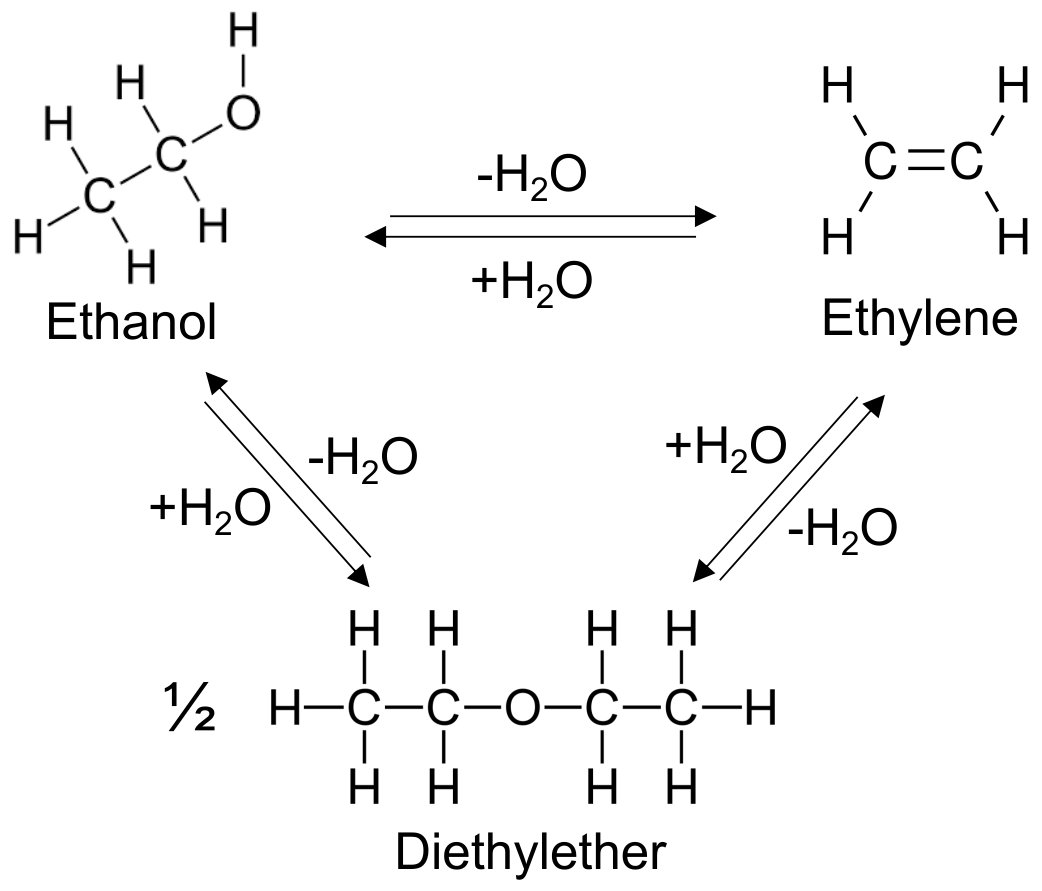
The primary reaction is a simple dehydration where ethanol loses one water molecule to form ethylene:
CH3CH2–OH → CH2=CH2 + H2O
This reaction is endothermic, requiring approximately 45 kJ/mol of heat input to proceed. The positive heat requirement means that reactors must continuously supply thermal energy to maintain conversion, unlike exothermic reactions that generate their own heat.
The reaction pathway is not entirely selective. A competing side reaction occurs where two ethanol molecules combine to form diethyl ether (DEE) plus water:
2 CH3CH2–OH → CH3CH2-O-CH2CH3 + H2O
The reaction pathway selectivity is strongly temperature-dependent and catalyst-dependent as outlined in the next section.
Catalyst Technology
The catalyst is the heart of the dehydration process, providing acid sites that activate ethanol molecules and facilitate water elimination. Gamma-alumina (γ-Al2O3) is the most widely used industrial catalyst due to its balance of activity, selectivity, thermal stability, and cost-effectiveness. Gamma-alumina possesses high surface area (150-300 m²/g) with both Lewis and Brønsted acid sites distributed across its porous structure.
The catalytic mechanism proceeds through ethanol adsorption onto acid sites, forming surface ethoxy intermediates that undergo beta-hydrogen elimination to release ethylene and water while regenerating the active site. The rate-limiting step is typically the carbon-hydrogen bond breaking during elimination, which explains the strong temperature dependence of reaction rates.
On traditional alumina catalysts, diethyl ether formation dominates at temperatures below approximately 250-280°C due to its lower activation energy and exothermic nature. As temperature increases above 350°C on alumina, the unimolecular ethylene-forming pathway becomes thermodynamically and kinetically favored, with ethylene selectivity approaching 95-100% at temperatures exceeding 400°C.
However, advanced zeolite catalysts fundamentally change this selectivity-temperature relationship. Dealuminated ZSM-5 zeolites achieve remarkable ethylene selectivities of 98-100% at temperatures as low as 220-280°C, significantly below the 400-450°C typically required for alumina systems. This superior low-temperature performance results from the zeolite's shape-selective pore structure that constrains transition state geometries, favoring unimolecular ethylene formation over bimolecular ether formation, combined with optimized acid site strength and distribution.
Catalyst deactivation occurs primarily through coke formation, where ethylene and unsaturated intermediates polymerize on acid sites to form carbonaceous deposits that block active sites and pore entrances. Water vapor in the feed partially mitigates coking by gasifying coke precursors. Deactivation progresses from the reactor inlet toward the outlet as a moving front, with catalyst lifetime ranging from months to years depending on operating severity. Regeneration involves controlled combustion of coke deposits in air at 500-600°C to restore activity.
Process Configuration
Reactor Design:
Industrial processes employ fixed-bed tubular reactors packed with catalyst pellets or extrudates. Two main configurations exist: adiabatic reactors arranged in series with inter-stage heating, or multi-tube isothermal reactors with external heat supply through a heated shell.
Operating Conditions:
- Temperature: 350-470°C (alumina catalysts), 220-350°C (zeolite catalysts)
- Pressure: 2-20 bar, typically 3-5 bar
- Weight hourly space velocity (WHSV): 0.5-6.0 h-¹, optimally 1.5-2.5 h-¹
- Steam dilution: 0.3:1 to 1.5:1 molar ratio steam:ethanol (optional)
Feed Preparation:
Liquid ethanol (anhydrous 99.5%+ or hydrated 93-96%) is vaporized and heated to reaction temperature. Steam may be added for dilution to suppress ether formation and serve as a heat carrier. Hydrated ethanol feeds are increasingly preferred because water helps suppress catalyst coking and eliminates the cost of molecular sieve dehydration required for anhydrous ethanol.?
Temperature Management:
Since the reaction is endothermic, temperature drops 40-80°C per adiabatic reactor stage as ethanol converts to ethylene. Multiple reactor stages with inter-stage heating maintain temperatures in the optimal range for high conversion and selectivity.
Performance Metrics
Conversion and Selectivity:
- Ethanol conversion: 95-99.9% per pass
- Ethylene selectivity: 94-100% (catalyst and temperature dependent)
- Overall ethylene yield: 94-98%
- Byproducts: Diethyl ether (1-4%), acetaldehyde (<1%), trace higher hydrocarbons
Productivity:
Advanced ZSM-5 catalysts achieve space-time yields exceeding 900-1500 g ethylene per kg catalyst per hour at optimized conditions, enabling compact reactor designs.
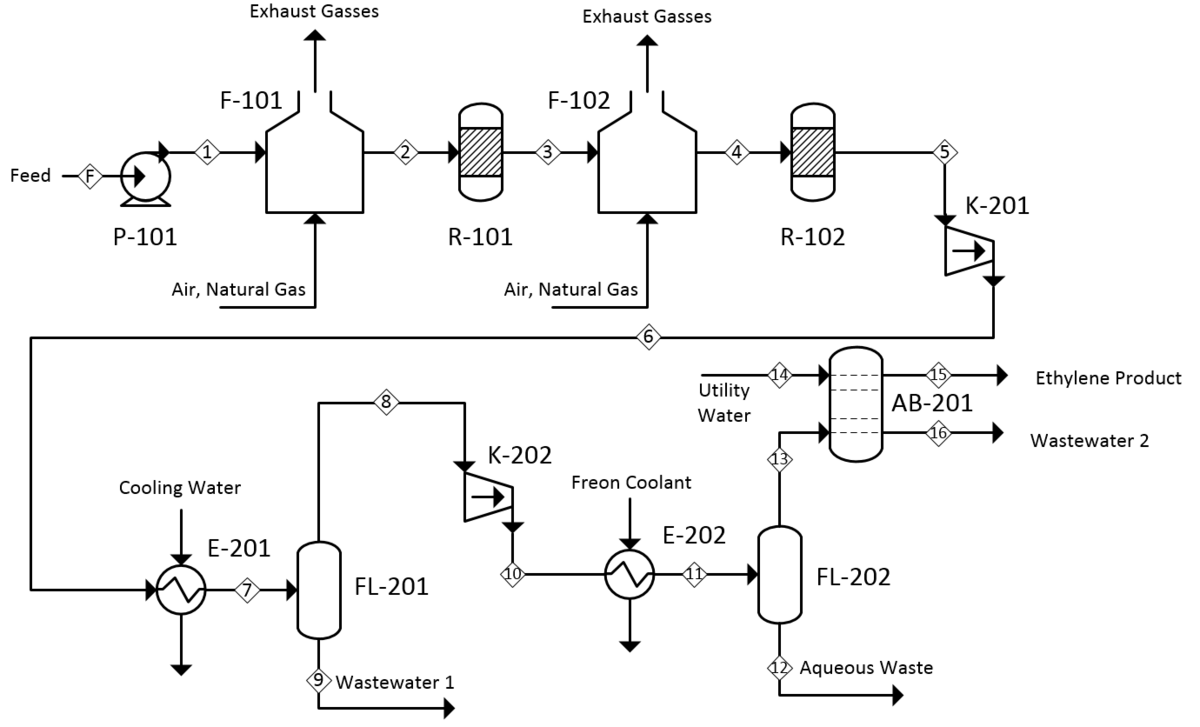
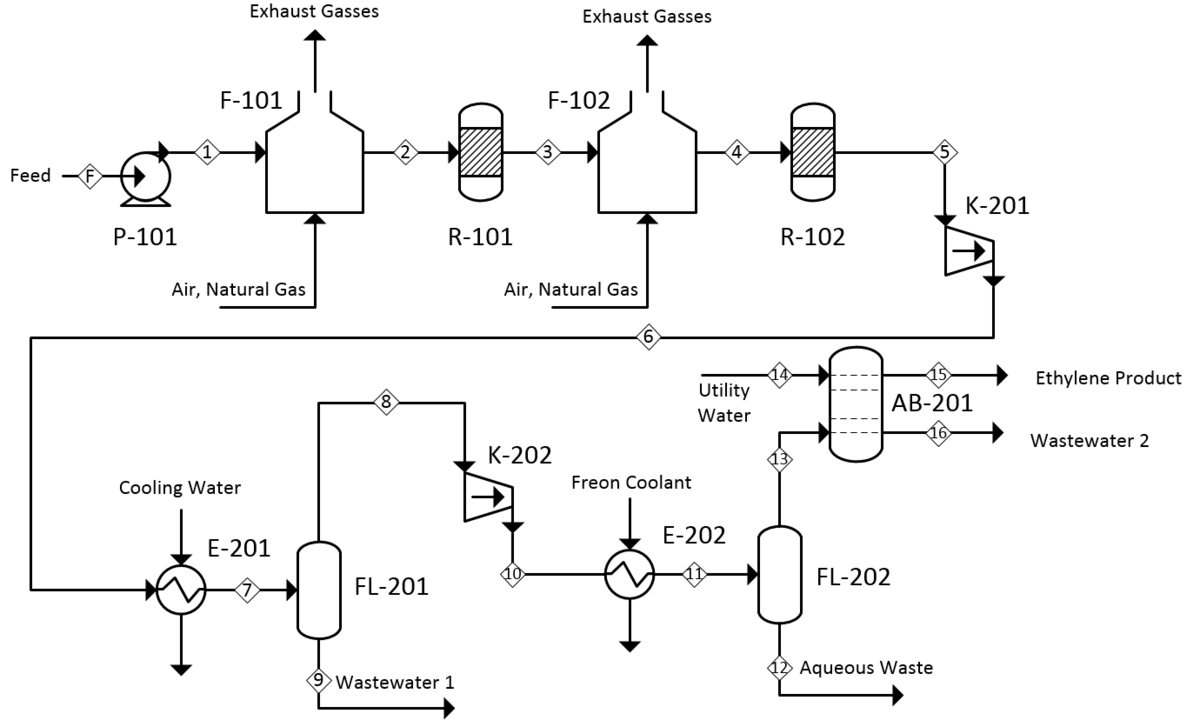
Product Recovery
Quench and Separation:
The reactor effluent (350-420°C) containing ethylene, water vapor, unreacted ethanol, and byproducts undergoes rapid cooling to condense water and ethanol while keeping ethylene gaseous. Gas-liquid separation recovers condensed aqueous ethanol for recycle to the reactor.
Purification Steps:
- Acid washing: Removes trace aldehydes and acidic impurities
- Drying: Molecular sieve beds reduce water content to <1 ppm
- Cryogenic distillation: Separates ethylene from ethane, ether, and residual ethanol to achieve polymer-grade purity of 99.9%+
The high-purity ethylene meets specifications for polyethylene production, while recovered ethanol and ether streams recycle to the reactor to maximize overall yield.
Energy and Economics
Energy Consumption:
Ethanol dehydration requires thermal energy for the endothermic reaction and heating to 350-470°C, plus electrical power for compression and refrigeration. Heat recovery from high-temperature reactor effluent (350-420°C) can generate steam or preheat feeds. Specific energy consumption figures are process-dependent and should be obtained from technology licensors or recent engineering studies.
Cost Structure:
Economics are dominated by ethanol feedstock costs due to the stoichiometric requirement of 1.68 kg ethanol per kg ethylene. A 2021 European study found profitability highly sensitive to the ethanol-to-ethylene price differential and regional factors including feedstock availability, energy costs, and carbon policies. Capital and operating costs vary substantially by location, capacity, and integration with existing facilities. Project-specific feasibility studies are essential for reliable cost estimation. A 2017 review reveals that existing first-generation bioethylene plants (referencing Braskem) do not function without subsidies as of 2017.
Process Variants
Feedstock Options:
- Anhydrous ethanol (99.5%+): Requires molecular sieve dehydration; eliminates feed water
- Hydrated ethanol (93-96%): Lower cost; water helps suppress coking; increasingly preferred
Reactor Configurations:
- Adiabatic fixed beds with furnace heating: Most common; simpler design
- Isothermal multi-tubular reactors: Better temperature control; higher capital cost
- Swing reactors: Enable continuous operation during catalyst regeneration cycles
Integration:
Processes may be integrated with upstream bioethanol production (sugarcane mills, corn ethanol plants) or downstream polyethylene production to optimize energy integration and logistics.
Proprietary Technologies
Chematur E2E Technology (1980s): Chematur Engineering AB developed an ethanol-to-ethylene (E2E) dehydration process based on technology originally created by American Halcon Scientific Design, Inc. in the 1980s, employing proprietary Syndol catalysts composed primarily of Al2O3-MgO/SiO2 (alumina-magnesia on silica support) in a configuration of four adiabatic tubular reactors arranged in series. At least one plant has built in Egypt and was operating as of 2024 since commissioning in 2014.
Petron Scientech K-SEETSM (1980s): Petron Scientech Inc. (PSI), a subsidiary of KBR, offers the K-SEET? (Kellog-Scientech Ethanol-to-Ethylene Technology) process for catalytic dehydration of ethanol to polymer-grade ethylene, based on technology originally developed by M.W. Kellogg Company in the 1980s and later enhanced by Scientech. The process employs proprietary fixed-bed catalyst systems in adiabatic reactors operating at moderate temperatures and pressures, designed for flexible capacity ranging from small-scale plants to large commercial facilities producing 100,000+ tons per year of ethylene. Petron Scientech has significantly more commercial experience than publicly acknowledged as revealed by the company's experience credentials.
Braskem EtE EverGreen™ (2010): Braskem developed and operates the world's first industrial-scale ethanol dehydration plant at its Triunfo petrochemical complex in Rio Grande do Sul, Brazil, which began production on September 24, 2010, with an initial capacity of 200,000 tons per year of bio-based ethylene from sugarcane bioethanol. The proprietary catalytic dehydration process converts renewable ethanol to polymer-grade ethylene using gamma-alumina-based catalysts in fixed-bed reactors, enabling production of I'm green™ bio-based polyethylene that isclaimed to avoid approximately 3 tons of CO2 per ton of product compared to fossil-based alternatives . Following successful commercial operation and a US$87 million investment, Braskem expanded the plant capacity by 30% to 260,000 tons per year in June 2023, and partnered with Lummus Technology in 2022 to globally license the technology as EtE EverGreen™ for deployment in North America and Asia.
TechnipEnergies Hummingbird® (2013): a proprietary ethanol-to-ethylene dehydration technology originally developed by BP Chemicals and unveiled in 2013, subsequently acquired by Technip (now TechnipEnergies) in June 2016. The technology employs a proprietary heteropolyacid (HPA) supported catalyst that operates at lower temperatures and higher pressures than conventional alumina-based processes, achieving ultra-high selectivity of over 99% for converting bioethanol to polymer-grade ethylene. Hummingbird® distinguishes itself through its nearly stoichiometric conversion efficiency that eliminates the need for ethylene fractionation columns, reducing capital costs and process complexity. The technology achieved its first commercial application in 2020 when LanzaTech selected Hummingbird® for integration with its Alcohol-to-Jet (ATJ) process at the LanzaJet Freedom Pines biorefinery in Soperton, Georgia, producing 10 million gallons per year of sustainable aviation fuel starting in 2022, with the demonstration plant accumulating over 36,000 hours of operation. TechnipEnergies continues to license Hummingbird® technology and supply its proprietary catalyst for commercial applications targeting sustainable aviation fuel and bio-based chemicals production.
Axens ATOL® (2014): IFPEN/Axens/Total launched ATOL® technology in 2014 with ATO 201 catalyst claiming superior economics through simplified purification and high selectivity. Two fixed bed adiabatic reactors, operating at 400–500 °C, are used in this process which produces 50 000–400 000 t a−1 ethylene. As per Sep 2023 ATOL® commercial bulletin, there are 6 references in the United States, Japan, Brazil and South Korea for both polymer and Sustainable Aviation Fuel (SAF) applications as Atol® technology is also the first step of Jetanol™ process suite (Alcohol To Jet) for the conversion of renewable/low carbon alcohols into SAF.
Tianjin University Ethanol Dehydrogenation (2025): Xinjiang Tianye Huixiang New Materials Co., Ltd. commissioned the largest single-train coal-based ethanol-to-ethylene unit (104,000 t/y) using patented technology domestically developed by Tianjin University + BPE Engineering- representing China's push for non-petroleum olefins self-sufficiency.
References
- Brito R.S.N. Controle do Processo de Desidratação do Etanol a Eteno (Feb 2018). Master's thesis, Universidade Federal do Rio de Janeiro, Rio de Janeiro, Brazil.
- Narin A.A. & Ghassan J.H. Kinetic Study of Ethanol Dehydration to Ethylene and Diethyl Ether in Catalytic Packed Bed Reactor Over ZSM-5 Catalyst (Jun 1, 2003). Diyala Journal of Engineering Sciences 16(2). DOI: 10.24237/djes.2023.16203
- Hiromichi A., Jun-Ichiro T., Yasukazu S., & Yukio Y. Ethanol dehydration on alumina catalysts: I. The thermal desorption of surface compounds (Oct 1967). Journal of Catalysis, 9(2):146-153. ISSN 0021-9517. DOI 10.1016/0021-9517(67)90193-5
- Chung-Yen W., & Ho-Shing W. Ethylene Formation from Ethanol Dehydration Using ZSM-5 Catalyst (Aug 2017). ACS Omega 2(8):4287–4296. DOI: 10.1021/acsomega.7b00680
- Ying Z., Zhaosheng J., & Wei S. Catalytic Dehydration of Ethanol to Ethylene (Oct 2011). DGMK Conference October 4-6, 2011, Dresden, Germany
- Inventors: Vieira M.A., Moura A.G., & Oliveira A.M. United States Patent US20250034062A1: Fixed bed reactors and processes for dehydration of alcohols. Jul 12, 2024: Application filed by Braskem S.A.
- Mitsuo K., Takahiro A., & Yukio A.. United States Patent US4302357A: Catalyst for production of ethylene from ethanol. Apr 23, 1980: Application filed by Nikki Kagaku KK (Jul 2006). THE ECONOMIC FEASIBILITY OF ETHANOL PRODUCTION FROM SUGAR IN THE UNITED STATES. USDA
- Poppick H., Fulk E., & Smith S. Ethanol to Ethylene (Mar 13, 2015). Cornell University
- Mohsenzadeh A., Zamani A., Taherzadeh M.J. Bioethylene Production from Ethanol: A Review and Techno-economical Evaluation (Mar 31, 2017). ChemBioEngReviews, 4(2):75-91, April 2017. DOI: 10.1002/cben.201600025
- French/Belgian partners launch Atol technology for bio-ethylene productions (Mar 2014). Plastics & Rubber Asia
- Bioolefins (Accessed: Dec 12, 2025). Axens
- Braskem inaugura fábrica de eteno verde em Triunfo-RS e assume a liderança global em biopolímeros (Sep 27, 2010). Braskem
- Braskem and Lummus partnership: the next chapter for Green Ethylene technology (Apr 27, 2022). Braskem
- Lummus to License Braskem Technology for Green Ethylene Projects. Advanced Biofuels USA
- (Jul 27, 2023). Braskem expands its biopolymer production by 30% following an investment of US$ 87 million (Nov 30, 2021). Braskem
- Yanbin D. News: China's ethanol-to-ethylene technology has achieved a major breakthrough, with the largest unit in the country successfully operating (Jul 30, 2025). Tianjin University
- Qian S. Shaanxi Daily: Shaanxi helps put into production the largest single ethanol dehydration and ethylene production unit in China (Jul 28, 2025). Shaanxi Province Website
- Xinjiang Tianye's 250,000-ton/year ethanol unit successfully put into production (Sep 18, 2024). China Science News
- Inventor: ???, ??, ??, ??, ???. Chinese Patent CN115055132A: Production process and device for preparing ethylene by ethanol dehydration. Jun 15, 2022: Application filed by Tianjin University
- Tullo A.H. BP Recommits To Chemicals (Nov 25, 2013). C&EN
- Technip acquires Hummingbird technology from BP Chemicals (Jun 15, 2016). Fibre2Fashion
- Hummingbird® ethanol-to-ethylene technology (Accessed: Dec 12, 2025). Technip Energies
- Hummingbird ethanol-to-ethylene technology case study (Jun 2024). Technip Energies
- Hummingbird Flysheet (Nov 2023). Technip Energies
- TechnipFMC’s Hummingbird® Ethylene Technology Selected by LanzaTech for LanzaJet Sustainable Aviation Fuel Biorefinery (Jul 17, 2020). TechnipFMC
- Press Release: Technip Energies announces first Hummingbird® catalyst supply agreement for LanzaJet biorefinery (Dec 5, 2021). LANZAJET
- Bio ethylene/ethene (Accessed: Dec 12, 2025). IBI Chematur
- Chematur Engineering AB Linkedin Post. TCI SANMAR CHEMICALS SAE built a Bio-Ethylene plant based on our E2E technology in Port Said, Egypt, and the plant has been in smooth operation since it was commissioned in 2014 (2024). Linkedin
- Burridge E. KBR and Petron in Technology Alliance (Aug 10, 2021). CHEManager
- Petron Scientech and Mitsui & Co. (USA), Inc. to Jointly Explore Development of Bio-Based Ethylene Infrastructure to Manufacture Bio PET (Dec 13, 2022). Petron Scientech Inc.
- Lane J. Competitive Edge: Petron Scientech, Inc. – From Ethanol to Ethylene, Ethylene Oxide and Ethylene Glycols (Mar 26, 2021). Advanced Biofuels USA (Retrieved from the Web Archive)
- Experience Credentials (1988-2022) - Ethanol to Green Ethylene Projects. Petron Scientech Inc. (Retrieved from the Web Archive)