Introduction
In industry, olefin plants (steam crackers) are used for the production of ethylene and propylene, which are the main feedstocks for the production of polyolefins, which account for 50–60% of all commercial organic chemicals. A Steam Cracker (SC), aka Steam Cracker Unit (SCU), is a facility in which a hydrocarbon feedstock is thermally cracked through the use of steam in the absence of oxygen in a bank of pyrolysis furnaces (Fig. 1) to produce lighter hydrocarbons[1,2].
Figure 1 - Pyrolisys Furnaces in a Steam Cracker

Feedstock Sources
The feed for the steam cracking unit can be gaseous or liquid hydrocarbons, such as ethane, propane, butane, naphtha, condensate and (more rarely) gas oils[3].
- Ethane
Usually recovered from natural gas fields, mainly in the USA.
- Propane/Butane
Recovered from gas fields in the Middle East, Texas, etc. Kuwait has a large butane recovery system. Propane and Butanes also originate from LNG plants.
- Refinery Naphtha
C5 to C7 paraffins based on low octane naphtha from refineries.
- Condensate
Similar to low octane naphtha but originating from natural gas and crude oil well head production.
- Light and Heavy, refinery-based Gasoils
Atmospheric Gas Oil (200°C to 350°C cut) and Vacuum Gas Oil (350°C to 550°C cut).
Steam Cracking Process
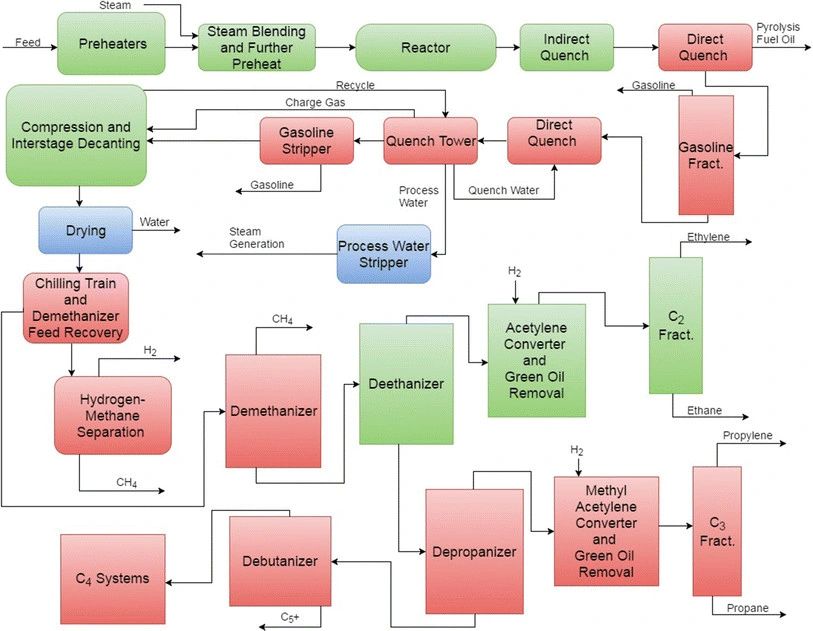
The total process of pyrolysis of hydrocarbons is organized in two sections: Pyrolysis and Separation as show in Fig. 2 [2].
Figure 2 - Schematic representation of pyrolysis of gasoline and product separation
1 - pyrolysis reactor (furnace), 2 - cooling tube heat exchanger, 3 - steam generator, 4 - primary fractionator, 5 - cooling distillation column, 6 - gas cleaning, 7 - drying column, 8 - low temperature cooling, 9 - separation of methane and hydrogen, 10 – column for de-methanation, 11 – column for de-ethanation, 12 - hydrogenation of acetylene, 13 - separation of ethylene, 14 – column for de-propanation, 15 - hydrogenation of methylacetylene, 16 - the separation of propylene, 17 - columns for de-butanation, 18 - columns for de-pentanation, 19 - separation of pyrolysis gasoline
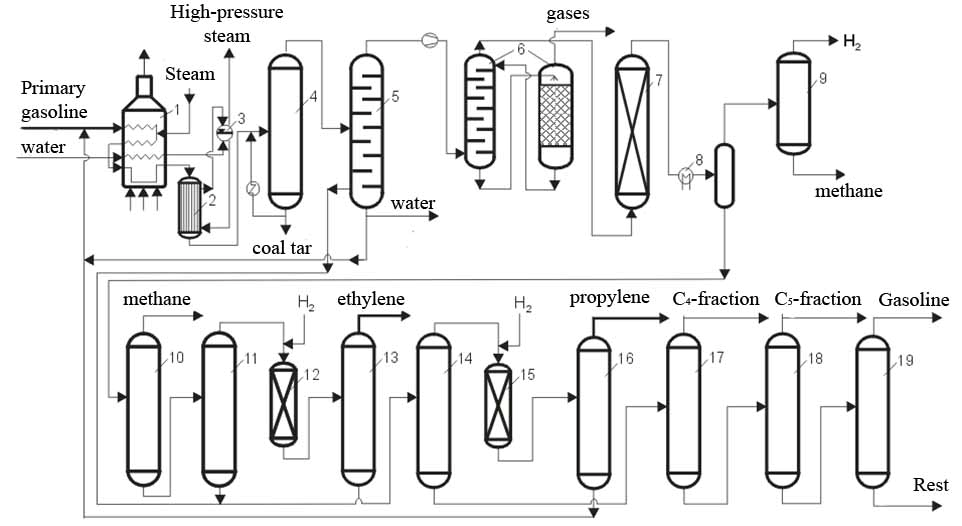
Steam cracking is a non-catalyzed process of thermal decomposition of saturated hydrocarbons in the presence of steam added to the feed of the reactor called Pyrolysis Furnace (Fig. 3), whereby commercial steam cracking of hydrocarbons is performed almost exclusively in tubular reactors. The role of steam is to prevent air to enter into the reactor and to form an explosive mixture with the cracked gases. Steam also acts as a diluent inhibiting carbonisation and limiting the formation of side products[2].
Figure 3 - Schematic diagram of typical steam cracking furnace[4]
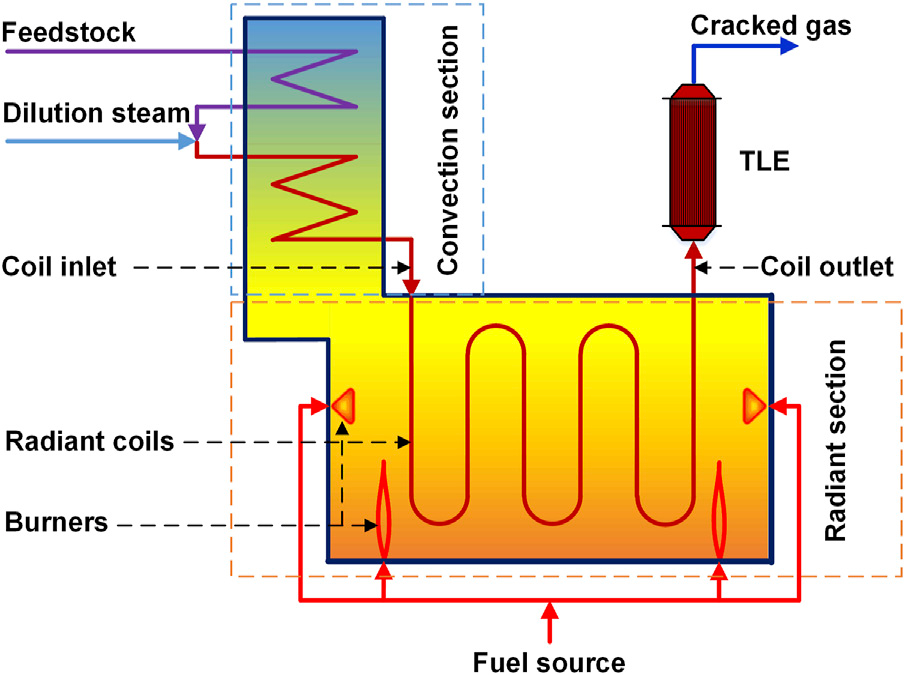
Pyrolysis is performed at very high temperatures of 750 to 900°C and at slightly above atmospheric pressure. High temperature enables the reaction to be carried out in a very short time, preventing a higher proportion of side reactions, especially the formation of coke. The retention time in the reactor (reaction furnace) is in the range from 0.1 to 10 seconds depending on the feedstock. In modern cracking furnaces, the residence time is reduced to milliseconds to improve yield (to avoid undesirable overcracking and coke formation), resulting in gas velocities faster than the speed of sound. After the cracking temperature has been reached, the gas is quickly quenched to stop the reaction in a transfer line heat exchanger (TLE) or inside a quenching header using quench oil.[2]
Figure 4 - Steam Cracking schematic representation
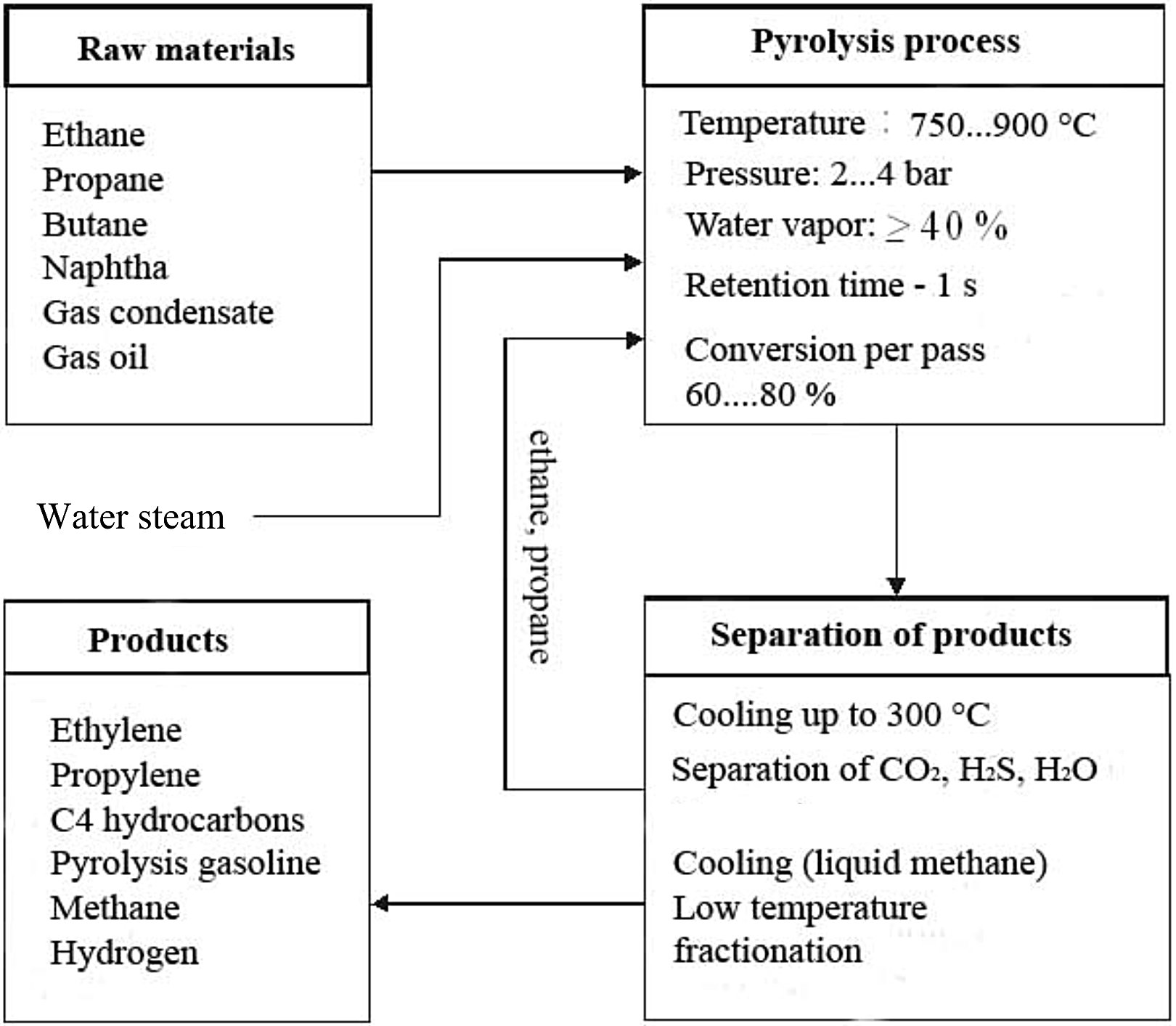
Butadiene, butylene, aromatics, and benzene-rich pyrolysis gasoline are produced when heavy liquid feeds such as naphtha and gas oils are used as feedstock, whereas methane, ethylene, propylene, and benzene can be produced from any optional feedstocks[1] as depicted in Fig 4. The process also results in the slow deposition of coke, a form of carbon, on the reactor walls. This degrades the efficiency of the reactor, so reaction conditions are designed to minimize this. Nonetheless, a steam cracking furnace can usually only run for a few months at a time between de-cokings.[2]
The products from steam cracking include a mixture of C1-C4 hydrocarbons and are separated in a complex process involving cooling, compression, absorption, drying, refrigeration, fractionation and selective hydrogenation. Some of the columns are shown in Fig. 5. A very low temperature (−114°C) is required for the demethanization process. Methane and hydrogen are separated at cryogenic temperatures. A large distillation column with 120–180 trays with a high reflux ratio is used for the separation of ethylene and ethane (C2 compounds). The resulting ethane, methane and hydrogen are separated and commonly used as a feedstock in other processes or as fuel for the reactor (the pyrolytic furnace). Extractive distillation or hydrogenation is used to remove the produced acetylene. Propylene and propane are re-boiled at around 80°C with the quench water and separated in a C3 splitter. The ethylene and propylene refrigeration systems can be operated at low temperatures (between −110°C and −150°C) for cooling and high pressure (15–30 bar) for compression. Remaining ethane, propane and part of non-reacted initial hydrocarbons are returned into the process. Finally, the C4 compounds and aromatic gasoline are separated[1,2].
Figure 5 - Separation Columns in a Steam Cracking Plant[2]

1 - A debutaniser which separates the C4 hydrocarbons from the C1-C3 hydrocarbons, 2 - A depropaniser which separates out the C3 hydrocarbons, 3 - A deethaniser which separates out the C2 hydrocarbons, 4 - A demethaniser which separates out the methane, 5 - A C3 splitter which separates propene from propane, 6 - A C2 splitter which separates ethene from ethane.
Cracking Yields
The more paraffinic the feedstock, the higher the ethylene yields and the greater the value of the co-products. Typical cracking yields for various feedstock types are presented in Table 1, assuming maximum recycle of the light alcanes (ethane, propane, butane)[3]. The highest yield of ethylene is obtained by the dehydrogenation (pyrolysis) of ethane (80%), but due to insufficient quantity, alternative feedstocks are also used in the production of olefins[2].
Table 1 - Feedstock-dependent Cracking Yields (in weight-%)
| PRODUCTS |
FEEDSTOCK |
| |
Ethane |
Propane |
Butane |
Naphtha |
AGO |
VGO |
| Hydrogen (95%) |
8.8 |
2.3 |
1.6 |
1.5 |
0.9 |
0.8 |
| Methane |
6.3 |
27.5 |
22 |
17.2 |
11.2 |
8.8 |
| Ethylene |
77.8 |
42 |
40 |
33.6 |
26 |
20.5 |
| Propylene |
2.8 |
16.8 |
17.3 |
15.6 |
16.1 |
14 |
| Butadiene |
1.9 |
3 |
3.5 |
4.5 |
4.5 |
5.3 |
| Other C4's |
0.7 |
1.3 |
6.8 |
4.2 |
4.8 |
6.3 |
| C5 to 200°C PyGas |
1.7 |
6.6 |
7.1 |
18.7 |
18.4 |
19.3 |
| Benzene |
0.9 |
2.5 |
3.0 |
6.7 |
6.0 |
3.7 |
| Toluene |
0.1 |
0.5 |
0.8 |
3.4 |
2.9 |
2.9 |
| C9 Aromatics |
- |
- |
0.4 |
1.8 |
2.2 |
1.9 |
| Non Aromatics |
0.7 |
3.6 |
2.9 |
6.8 |
7.3 |
10.8 |
| Fuel Oil |
- |
0.5 |
1.7 |
4.7 |
18.1 |
25 |
The products obtained not only depend upon the composition of the feed, but also on the hydrocarbon-to-steam ratio, and on the cracking temperature and furnace residence time[1]. A higher cracking temperature (also referred to as severity) favors the production of ethene and benzene, whereas lower severity produces higher amounts of propene, C4-hydrocarbons and liquid products as shown in Table 2[2].
Table 2 - Product yields in weight-% from the steam cracking of naphtha under different severity conditions
Parameters →
Product ↓ |
Low severity: 1,000°K
Residence time: 0.5s |
High severity: 1,150°K
Residence time: 0.1s |
| Hydrogen |
1 |
1 |
| Methane |
15 |
18 |
| Ethylene |
19 |
32 |
| Propene |
16 |
13 |
| C4 hydrocarbons |
10 |
9 |
| Raw PyGas |
36 |
18 |
| Others |
3 |
9 |
Energy Aspects
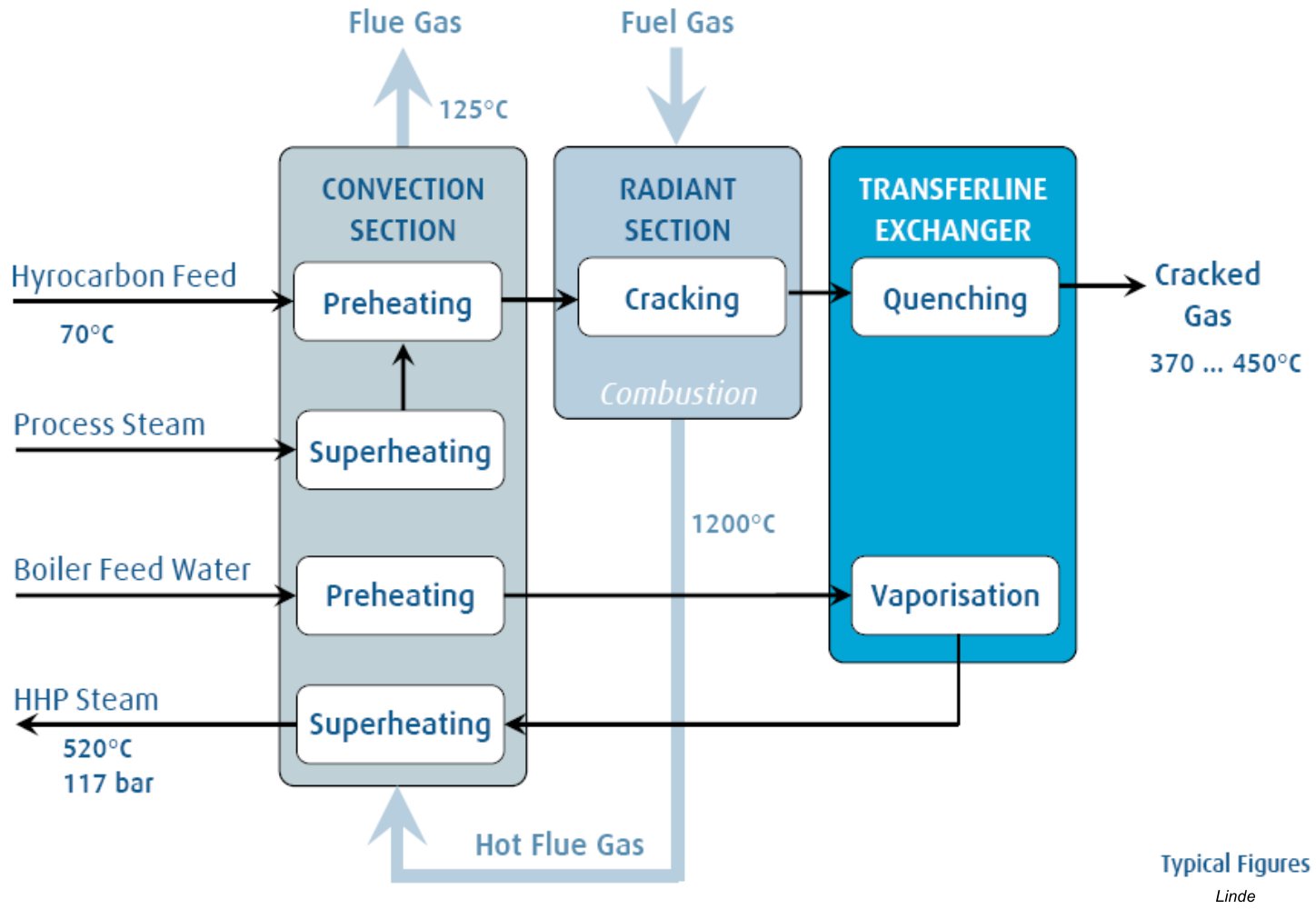
Whilst the fundamentals of the process have not changed in recent decades, improvements continue to be made to the energy efficiency of the furnace through integrated heating systems and heat recycling, ensuring that the cost of production is continually reduced. Therefore, high energy rates are needed[2]. Energy cost accounts for approximately 70% of production costs in typical ethane- or naphtha-based olefin plants[5]. This high energy demand is due to the endothermic characteristics of the cracking of the C–C bonds of the hydrocarbons [1].
Figure 6 - Integrated Heating System of Cracking Furnaces (Typical Values)[2]
References
- Gholami Z, Gholami F, Tišler Z, Vakili M. A Review on the Production of Light Olefins Using Steam Cracking of Hydrocarbons. Energies. 2021; 14(23):8190 and references comprised therein.
- Ante Jukic, Apr 2013, Petroleum Refining and Petrochemical Processes Production of Olefins – Steam Cracking of Hydrocarbons, University of Zagreb, Faculty of Chemical Engineering and Technology.
- Gerard B. Hawkins, 3rd Aug 2013, Ethylene Plant Considerations, slideshare.
- Ren, Yu & Liao, Zuwei & Yang, Yao & Sun, Jingyuan & Jiang, Binbo & Wang, Jingdai & Yang, Yongrong. (2022). Direct prediction of steam cracking products from naphtha bulk properties: Application of the two sub-networks ANN. Frontiers in Chemical Engineering. 4. 10.3389/fceng.2022.983035.
- Ren, T.; Patel, M.; Blok, K. Olefins from conventional and heavy feedstocks: Energy use in steam cracking and alternative processes. Energy 2006, 31 (4), 425−451.